Chemistry -- Oxidation
... Acids donate _____ and bases accept ____ H+ H+ proton(s) proton(s) In other reactions, substances donate or accept electrons The flow of electrons from one substance to the next is how batteries “make” electricity ...
... Acids donate _____ and bases accept ____ H+ H+ proton(s) proton(s) In other reactions, substances donate or accept electrons The flow of electrons from one substance to the next is how batteries “make” electricity ...
Chapter 40
... b) Energy is added to the atoms in a laser such that more electrons occupy a metastable higher energy state than are in a lower energy state. c) This occurs inside a laser when there are more higher energy photons than lower energy photons. d) This is the photon-electron process within an atom that ...
... b) Energy is added to the atoms in a laser such that more electrons occupy a metastable higher energy state than are in a lower energy state. c) This occurs inside a laser when there are more higher energy photons than lower energy photons. d) This is the photon-electron process within an atom that ...
C:\Documents and Settings\Travis D. Fridgen\My Documents
... For a one electron atom, such as hydrogen, the 3s, 3p and 3d orbitals are degenerate. However, for a many-electron atom the energy levels split. Briefly explain, referring to this figure, how penetration affects the energy splitting of the 3s, 3p and 3d orbitals for a manyelectron atom. From the dia ...
... For a one electron atom, such as hydrogen, the 3s, 3p and 3d orbitals are degenerate. However, for a many-electron atom the energy levels split. Briefly explain, referring to this figure, how penetration affects the energy splitting of the 3s, 3p and 3d orbitals for a manyelectron atom. From the dia ...
Unit_Chemistry_2_Ionic_Substances_and_Electrolysis
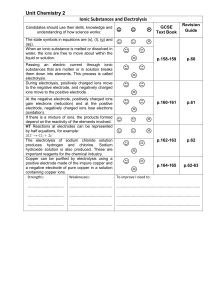
... Ionic Substances and Electrolysis Candidates should use their skills, knowledge and understanding of how science works: ...
... Ionic Substances and Electrolysis Candidates should use their skills, knowledge and understanding of how science works: ...
A COMPUTATIONAL STUDY OF -SCN
... 8. In the Surfaces window, click the Add button. Choose HOMO. Move the window out of the way, but leave it open. 9. On the menu bar, click Setup, and then Calculations. a. Calculate: Equilibrium Geometry with Hartree-Fock 3-21G should already be selected (both are default settings). b. Determine how ...
... 8. In the Surfaces window, click the Add button. Choose HOMO. Move the window out of the way, but leave it open. 9. On the menu bar, click Setup, and then Calculations. a. Calculate: Equilibrium Geometry with Hartree-Fock 3-21G should already be selected (both are default settings). b. Determine how ...
Computational Quantum Chemistry
... Comparison to Classical Methods Quantum models don’t necessarily need empirical parameters: applicable in principle to any molecule Quantum mechanics provides all information that can be knowable about a system (QM postulate). Often much more accurate and reliable. Computations can be vastly more t ...
... Comparison to Classical Methods Quantum models don’t necessarily need empirical parameters: applicable in principle to any molecule Quantum mechanics provides all information that can be knowable about a system (QM postulate). Often much more accurate and reliable. Computations can be vastly more t ...
A COMPUTATIONAL STUDY OF -SCN
... 8. In the Surfaces window, click the Add button. Choose HOMO. Move the window out of the way, but leave it open. 9. On the menu bar, click Setup, and then Calculations. a. Calculate: Equilibrium Geometry with Hartree-Fock 3-21G should already be selected (both are default settings). b. Determine how ...
... 8. In the Surfaces window, click the Add button. Choose HOMO. Move the window out of the way, but leave it open. 9. On the menu bar, click Setup, and then Calculations. a. Calculate: Equilibrium Geometry with Hartree-Fock 3-21G should already be selected (both are default settings). b. Determine how ...
o C
... The Law of Conservation of Matter and Energy says that matter can be neither created nor destroyed during chemical ...
... The Law of Conservation of Matter and Energy says that matter can be neither created nor destroyed during chemical ...
ET3034TUx -‐ 2.2.1 – Band Gap I: Electrons in Atoms
... It is not straightforward to quickly explain this principle, but I will give it a try. I will use a chemical picture to explain the nature of the band gap. You have to realize that I ...
... It is not straightforward to quickly explain this principle, but I will give it a try. I will use a chemical picture to explain the nature of the band gap. You have to realize that I ...
chapter 2
... 13. What two things are classified as pure substances?___ compounds _____ and ____ elements ______ 14. What is the difference between a homogeneous and heterogeneous mixture? _____________________ __ HO – looks uniform in composition; HE – you can see different parts ____________ 15. Describe each o ...
... 13. What two things are classified as pure substances?___ compounds _____ and ____ elements ______ 14. What is the difference between a homogeneous and heterogeneous mixture? _____________________ __ HO – looks uniform in composition; HE – you can see different parts ____________ 15. Describe each o ...
On the nature of chemical bonding in γ-boron
... nuclei. According to Born’s interpretation of Schroedinger’s wave equation, the famous Ψ2 represent only the probability where the electron in the orbital can be found; therefore, orbitals cannot function as the glue that holds together the nuclei… Molecular orbital theory is the most advanced theor ...
... nuclei. According to Born’s interpretation of Schroedinger’s wave equation, the famous Ψ2 represent only the probability where the electron in the orbital can be found; therefore, orbitals cannot function as the glue that holds together the nuclei… Molecular orbital theory is the most advanced theor ...
Kinds of Chemistry - Louisiana State University
... We have measured mass of proton: 1.66 x 10-24 g We have measured mass of electron: 1836 times lighter than proton We have measured charge of proton: +1.602 x 10-19 Coulombs We have measured charge of electron: -1.602 x 10-19 Coulombs We know protons are at the center of atom. Neutrons were found—fix ...
... We have measured mass of proton: 1.66 x 10-24 g We have measured mass of electron: 1836 times lighter than proton We have measured charge of proton: +1.602 x 10-19 Coulombs We have measured charge of electron: -1.602 x 10-19 Coulombs We know protons are at the center of atom. Neutrons were found—fix ...
Chemistry 1 Lectures
... Which of the following molecules have a dipole moment? H2O, CO2, SO2, and CH4 ...
... Which of the following molecules have a dipole moment? H2O, CO2, SO2, and CH4 ...
Document
... Energy depends on L and S, not on ML or MS. • (L, S, J, MJ) are good quantum numbers for heavy many-electron atoms with significant spin-orbit coupling (relativistic effect). Energy also depends on J. • For very heavy atoms, a j-j coupling is needed, where j = l + s for each electron. ...
... Energy depends on L and S, not on ML or MS. • (L, S, J, MJ) are good quantum numbers for heavy many-electron atoms with significant spin-orbit coupling (relativistic effect). Energy also depends on J. • For very heavy atoms, a j-j coupling is needed, where j = l + s for each electron. ...
CHEM1001 2012-J-2 June 2012 22/01(a) • Complete the following
... Calculate the mass of NH3 required to produce 140. g of water. The molar mass of H2O is: molar mass = [2 × 1.008 (H) + 16.00 (O)] g mol-1 = 18.016 g mol-1 Hence, the number of moles of water produced is: number of moles = mass / molar mass = (140. g) / (18.016 g mol-1) = 7.771 mol From the balanced ...
... Calculate the mass of NH3 required to produce 140. g of water. The molar mass of H2O is: molar mass = [2 × 1.008 (H) + 16.00 (O)] g mol-1 = 18.016 g mol-1 Hence, the number of moles of water produced is: number of moles = mass / molar mass = (140. g) / (18.016 g mol-1) = 7.771 mol From the balanced ...
File
... Solvation • The process of a solute dissolving in a solvent • Solute is added to solvent solvent particles attract solute particles bonds holding solute together break down solute becomes surrounded by solvent molecules • if the attraction between particles of the solute is stronger than thos ...
... Solvation • The process of a solute dissolving in a solvent • Solute is added to solvent solvent particles attract solute particles bonds holding solute together break down solute becomes surrounded by solvent molecules • if the attraction between particles of the solute is stronger than thos ...
O - FIU
... Selective membranes for toxic/waste and environmental control Artificial photosynthesis systems for clean energy Tiny robotic systems for healthcare and space exploration “Never has such a comprehensive technology promised to change ...
... Selective membranes for toxic/waste and environmental control Artificial photosynthesis systems for clean energy Tiny robotic systems for healthcare and space exploration “Never has such a comprehensive technology promised to change ...
Chemistry - Unit 6 What do you need to know?? This chapter is on
... Atoms are indestructible and unchangeable, so compounds, such as water and mercury calx, are formed when one atom chemically combines with other atoms. This was an extremely advanced concept for its time; while Dalton’s theory implied that atoms bonded together, it would be more than 100 years befor ...
... Atoms are indestructible and unchangeable, so compounds, such as water and mercury calx, are formed when one atom chemically combines with other atoms. This was an extremely advanced concept for its time; while Dalton’s theory implied that atoms bonded together, it would be more than 100 years befor ...
Document
... Notes and images from instructor’s resource CD for Chemistry: The Central Science, 9 th Edition, Brown, LeMay, Bursten & Burdge, © 2003, Pearson Education, One Lake Street, Upper Saddle River, NJ 07485 ...
... Notes and images from instructor’s resource CD for Chemistry: The Central Science, 9 th Edition, Brown, LeMay, Bursten & Burdge, © 2003, Pearson Education, One Lake Street, Upper Saddle River, NJ 07485 ...
Chemical Reactions
... – Chemical equations show the conversion of reactants (the molecules shown on the left of the arrow) into products (the molecules shown on the right of the arrow). • + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen re ...
... – Chemical equations show the conversion of reactants (the molecules shown on the left of the arrow) into products (the molecules shown on the right of the arrow). • + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen re ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.