Document
... 1. Explain what is wrong with the statement “My friend burned a piece of paper (a hydrocarbon) that had the final exam on it and it disappeared”. (Be sure to use a chemical equation, identify reactants and product(s) and include energy). ANSWER: The paper (CxHy) was burned with oxygen and the atoms ...
... 1. Explain what is wrong with the statement “My friend burned a piece of paper (a hydrocarbon) that had the final exam on it and it disappeared”. (Be sure to use a chemical equation, identify reactants and product(s) and include energy). ANSWER: The paper (CxHy) was burned with oxygen and the atoms ...
107 - Bossier Parish Community College
... 13. recognize the interrelationship of the structure of matter and its chemical and physical properties. (B,C) 14. differentiate between intensive and extensive properties. (B,C) 15. determine if a change in matter is physical or chemical. (B,C) 16. recognize and differentiate the characteristics o ...
... 13. recognize the interrelationship of the structure of matter and its chemical and physical properties. (B,C) 14. differentiate between intensive and extensive properties. (B,C) 15. determine if a change in matter is physical or chemical. (B,C) 16. recognize and differentiate the characteristics o ...
Worksheet 8 Notes - Department of Chemistry | Oregon State
... Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas involved protons—bases accept a proton and acid ...
... Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas involved protons—bases accept a proton and acid ...
Compounds & Moles
... As the number of oxygen atoms changes in oxyacids, so does the name (just like the oxyanions) 1 more oxygen = per_______ic acid Common form = _______ic acid 1 less oxygen = _______ous acid 2 less oxygens = hypo_______ous acid ...
... As the number of oxygen atoms changes in oxyacids, so does the name (just like the oxyanions) 1 more oxygen = per_______ic acid Common form = _______ic acid 1 less oxygen = _______ous acid 2 less oxygens = hypo_______ous acid ...
Honors Mid-Term Review Sheet
... 78. Define nonpolar bond and polar bond. 79. Tell if the following molecules contain nonpolar, polar, or ionic bonds. a. CH4 b. HCl c. H2O d. Li3N e. F2 80. Define dipole moment. 81. How many lone pairs of electrons are in the Lewis dot structure for H2O? 82. Draw the Lewis dot structures for the fo ...
... 78. Define nonpolar bond and polar bond. 79. Tell if the following molecules contain nonpolar, polar, or ionic bonds. a. CH4 b. HCl c. H2O d. Li3N e. F2 80. Define dipole moment. 81. How many lone pairs of electrons are in the Lewis dot structure for H2O? 82. Draw the Lewis dot structures for the fo ...
S3 Numeracy Booklets – Atomic Structure
... related to the atomic structure of elements. i.e. if you wanted to get the number 24 you could ask: What number is equal to the atomic mass of carbon + the atomic number of magnesium? Ans: atomic mass of carbon is 12; atomic number of magnesium is 12, ...
... related to the atomic structure of elements. i.e. if you wanted to get the number 24 you could ask: What number is equal to the atomic mass of carbon + the atomic number of magnesium? Ans: atomic mass of carbon is 12; atomic number of magnesium is 12, ...
Schrodinger models of the atom
... Orbitals are regions of space. The electrons are like a cloud of negative charge within that orbital. The electron shells proposed by Bohr are still used, but the electrons in each shell are not all equal in energy. The shell has subshells. The quantum mechanical model is a complex mathematical theo ...
... Orbitals are regions of space. The electrons are like a cloud of negative charge within that orbital. The electron shells proposed by Bohr are still used, but the electrons in each shell are not all equal in energy. The shell has subshells. The quantum mechanical model is a complex mathematical theo ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
Energy and Matter in Chemical Change Science 10
... • The latest experimental evidence has disproved Bohr’s idea of fixed energy levels • Now, they visualize electrons not as a particle, but as a cloud of negative charge. Rather than following little race tracks around the nucleus, they occupy the whole space all at once at different energy levels ...
... • The latest experimental evidence has disproved Bohr’s idea of fixed energy levels • Now, they visualize electrons not as a particle, but as a cloud of negative charge. Rather than following little race tracks around the nucleus, they occupy the whole space all at once at different energy levels ...
Chapter 7, Quantum Nos.
... For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, ...
... For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, ...
Chapter 4-2 The Quantum Model of the Atom
... orbitals of different shapes exist for a given value of n. The angular momentum quantum number, symbolized by l, indicates the shape of the orbital. The number of orbital shapes possible is equal to n. The values of l allowed are zero and all positive integers less than or equal to n-1. ...
... orbitals of different shapes exist for a given value of n. The angular momentum quantum number, symbolized by l, indicates the shape of the orbital. The number of orbital shapes possible is equal to n. The values of l allowed are zero and all positive integers less than or equal to n-1. ...
Besombes - International Conference on Quantum Dots (QD 2012)
... devices has dramatically reduced the number of atoms necessary to process and store one bit of information: An individual magnetic atom would represent the ultimate size limit for storing and processing information. With semiconductor quantum dots (QDs) doped with Mn atoms, the probing of a single a ...
... devices has dramatically reduced the number of atoms necessary to process and store one bit of information: An individual magnetic atom would represent the ultimate size limit for storing and processing information. With semiconductor quantum dots (QDs) doped with Mn atoms, the probing of a single a ...
Chp7,Quantum_Num
... For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, ...
... For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, ...
Document
... Atoms that share electrons can also form giant structures or macromolecules. Diamond and graphite (forms of carbon) and silicon dioxide (silica) are examples of giant covalent structures (lattices) of atoms. All the atoms in these structures are linked to other atoms by strong covalent bonds and so ...
... Atoms that share electrons can also form giant structures or macromolecules. Diamond and graphite (forms of carbon) and silicon dioxide (silica) are examples of giant covalent structures (lattices) of atoms. All the atoms in these structures are linked to other atoms by strong covalent bonds and so ...
Atomic Structure Zumdahl Chemistry Chapter 7
... at which the electron is found from the nucleus. Schrodinger’s equations are beyond this class, but describe the probability of finding an electron at a given position around the nucleus. When graphed it gives us the shapes and positions of our electron orbitals. An orbital as a three-dimensional el ...
... at which the electron is found from the nucleus. Schrodinger’s equations are beyond this class, but describe the probability of finding an electron at a given position around the nucleus. When graphed it gives us the shapes and positions of our electron orbitals. An orbital as a three-dimensional el ...
makeup2
... likely to react chemically to form ions which have a charge of (A) +2e (B) +e (C) -e (D) -2e 14. The formula of the compound ammonium carbonate is (A) NH4CO3 (B) NH4CO4 (C) NH4HCO3 (D) (NH4)2CO3 15. Which statement regarding the properties of elements arranged in the Periodic Table is correct? (A) A ...
... likely to react chemically to form ions which have a charge of (A) +2e (B) +e (C) -e (D) -2e 14. The formula of the compound ammonium carbonate is (A) NH4CO3 (B) NH4CO4 (C) NH4HCO3 (D) (NH4)2CO3 15. Which statement regarding the properties of elements arranged in the Periodic Table is correct? (A) A ...
Electrons in Atoms blank guide
... condensed electron configuration – the e- config. of an atom that shows the number of electrons present in each energy level (listed vertically) electron dot formula or Lewis Dot diagrams: shows the arrangement of valence (outer shell) electrons around an atomic symbol.Take into account the electro ...
... condensed electron configuration – the e- config. of an atom that shows the number of electrons present in each energy level (listed vertically) electron dot formula or Lewis Dot diagrams: shows the arrangement of valence (outer shell) electrons around an atomic symbol.Take into account the electro ...
Balancing Chemical Equation Practice.docx
... Step 2: Draw out what each compound looks like by “chaining” the atoms/ions together. Remember that polyatomic ions act as a unit—they never come apart! Notice that there may not be the same number of atoms of each element. Reactants Products ...
... Step 2: Draw out what each compound looks like by “chaining” the atoms/ions together. Remember that polyatomic ions act as a unit—they never come apart! Notice that there may not be the same number of atoms of each element. Reactants Products ...
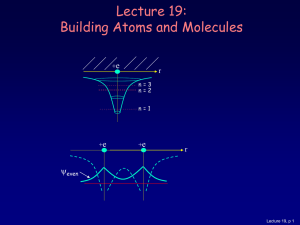
Quantum mechanics and electron structure
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstract mathematical model remains a fascinat ...
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstract mathematical model remains a fascinat ...
coppin state college
... Dr. Alfred N. Amah This examination consists of 38 multiple choice questions with five possible responses. Read each question carefully and choose the best response. There is only one correct response for each question. You are to answer all questions in this examination. 1. What method is used to d ...
... Dr. Alfred N. Amah This examination consists of 38 multiple choice questions with five possible responses. Read each question carefully and choose the best response. There is only one correct response for each question. You are to answer all questions in this examination. 1. What method is used to d ...
Solutions to the exam itself are now available.
... would be spherical, and if you circled these, you were not penalized as long as you also circled Si and Cl.) (f) The Cr4+ ion in the compound CrO2 is exploited in magnetic audio and video recording tape. The electron configuration of Cr4+ is [Ar] 3d2. The question here is simple: which four electron ...
... would be spherical, and if you circled these, you were not penalized as long as you also circled Si and Cl.) (f) The Cr4+ ion in the compound CrO2 is exploited in magnetic audio and video recording tape. The electron configuration of Cr4+ is [Ar] 3d2. The question here is simple: which four electron ...
Atomic Structure and Periodic Trends
... • Each orbital can hold a maximum of 2 electrons of opposite spin. • Pauli exclusion principle states that no two electrons in an atom can have the same set of 4 quantum numbers ...
... • Each orbital can hold a maximum of 2 electrons of opposite spin. • Pauli exclusion principle states that no two electrons in an atom can have the same set of 4 quantum numbers ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.