Tests
... research progress takes a lot of time and money some scientists are lazy and steal the ideas of others research progress occurs in small steps, so many people are needed ...
... research progress takes a lot of time and money some scientists are lazy and steal the ideas of others research progress occurs in small steps, so many people are needed ...
AP Chemistry Curriculum Map - Belle Vernon Area School District
... properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.1 – Explain how the periodicity of chemical properties led to the arrangement of elements on the periodic table. Compare and/or predict the properties (e.g., electron affinity, ionizatio ...
... properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.1 – Explain how the periodicity of chemical properties led to the arrangement of elements on the periodic table. Compare and/or predict the properties (e.g., electron affinity, ionizatio ...
lecture ch1-3 chem161pikul
... Mental picture that explains observed laws Tentative explanation of data Make predictions Leads to further tests Go to laboratory and perform experiments ...
... Mental picture that explains observed laws Tentative explanation of data Make predictions Leads to further tests Go to laboratory and perform experiments ...
CH4 Student Revision Guides pdf | GCE AS/A
... Like alkanes the structure is examined for the longest straight-chain carbon chain. The name is based on the hydrocarbon with the same number of C-atoms as the longest continuous carbon chain that contains the double bond. The lowest number is used to show the position of the double bond. The ending ...
... Like alkanes the structure is examined for the longest straight-chain carbon chain. The name is based on the hydrocarbon with the same number of C-atoms as the longest continuous carbon chain that contains the double bond. The lowest number is used to show the position of the double bond. The ending ...
AP* Chemistry: 2008 Released Multiple Choice Exam
... 17. An oxidation-reduction reaction that is also a synthesis reaction ...
... 17. An oxidation-reduction reaction that is also a synthesis reaction ...
The science of chemistry is concerned with the composition
... extremely toxic if breathed into the lungs. It has been responsible for many cases of human poisoning. In other respects mercury vapor behaves much like any gas. It is easily compressible. Even when quite modest pressures are applied, the volume decreases noticeably. It is also much less dense than ...
... extremely toxic if breathed into the lungs. It has been responsible for many cases of human poisoning. In other respects mercury vapor behaves much like any gas. It is easily compressible. Even when quite modest pressures are applied, the volume decreases noticeably. It is also much less dense than ...
The science of chemistry is concerned with the
... extremely toxic if breathed into the lungs. It has been responsible for many cases of human poisoning. In other respects mercury vapor behaves much like any gas. It is easily compressible. Even when quite modest pressures are applied, the volume decreases noticeably. It is also much less dense than ...
... extremely toxic if breathed into the lungs. It has been responsible for many cases of human poisoning. In other respects mercury vapor behaves much like any gas. It is easily compressible. Even when quite modest pressures are applied, the volume decreases noticeably. It is also much less dense than ...
ض ( ا ء ا ط ك ا رر 108 1) -
... Select the ionic compound formed from Aluminum, Al and oxygen, O? Al2O3 B Al3O2 C AlO2 D Al2O2 Write the formula for the ionic compound formed from pair of elements (Mg and O) ...
... Select the ionic compound formed from Aluminum, Al and oxygen, O? Al2O3 B Al3O2 C AlO2 D Al2O2 Write the formula for the ionic compound formed from pair of elements (Mg and O) ...
Unit 4 - Calculations and Chemical Reactions
... 4.6 Classification of Chemical Reactions There is no comprehensive classification scheme that would accommodate all known chemical reactions. One approach is to classify reactions into four types: combination, decomposition, single replacement and double replacement reactions. I) Combination Reacti ...
... 4.6 Classification of Chemical Reactions There is no comprehensive classification scheme that would accommodate all known chemical reactions. One approach is to classify reactions into four types: combination, decomposition, single replacement and double replacement reactions. I) Combination Reacti ...
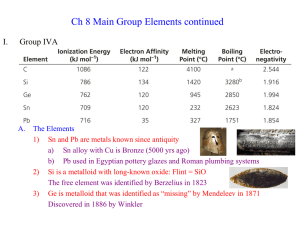
InorgCh8.2
... e) At2 in 1940 during particle collisions (radioactive with ½ life = only 8 hours) 3) Chemistry dominated by ready reduction to Xa) Excellent oxidizing agents (is reduced itself); F2 is best of all elements b) F is the most electronegative element, decreases down the group c) I2 has the highest Lond ...
... e) At2 in 1940 during particle collisions (radioactive with ½ life = only 8 hours) 3) Chemistry dominated by ready reduction to Xa) Excellent oxidizing agents (is reduced itself); F2 is best of all elements b) F is the most electronegative element, decreases down the group c) I2 has the highest Lond ...
AS Specification pdf | AS/A level
... This section outlines the knowledge, understanding and skills to be developed by learners studying AS Chemistry. Learners should be prepared to apply the knowledge, understanding and skills specified in a range of theoretical, practical, industrial and environmental contexts. It is a requirement of ...
... This section outlines the knowledge, understanding and skills to be developed by learners studying AS Chemistry. Learners should be prepared to apply the knowledge, understanding and skills specified in a range of theoretical, practical, industrial and environmental contexts. It is a requirement of ...
JSUNIL TUTORIAL , SAMASTIPUR, BIHAR
... respectively. The ratio of oxygen combining with 12 gm of Carbon is 16: 32 or, 1:2 which is in a simple ratio Dalton’s explanation for the law of conservation of mass and the law of definite proportions In 1803, A British school teacher John Dalton provided the basic theory about the nature of matte ...
... respectively. The ratio of oxygen combining with 12 gm of Carbon is 16: 32 or, 1:2 which is in a simple ratio Dalton’s explanation for the law of conservation of mass and the law of definite proportions In 1803, A British school teacher John Dalton provided the basic theory about the nature of matte ...
Title Decomposition studies of isopropanol in a
... consumption at the initial stages of reaction. Decomposition and pyrolysis occurs through dehydration and hydrogen elimination reactions, in addition to the usual simple C–C or C-H bond fissions typical of hydrocarbons. The relative significance of each of these processes affect the radical pool pop ...
... consumption at the initial stages of reaction. Decomposition and pyrolysis occurs through dehydration and hydrogen elimination reactions, in addition to the usual simple C–C or C-H bond fissions typical of hydrocarbons. The relative significance of each of these processes affect the radical pool pop ...
Inorganic Chemistry
... be nearly this long. This book is not a survey of the literature or a research monograph. It is a textbook that is intended to provide the background necessary for the reader to move on to those more advanced resources. In writing this book, I have attempted to produce a concise textbook that meets ...
... be nearly this long. This book is not a survey of the literature or a research monograph. It is a textbook that is intended to provide the background necessary for the reader to move on to those more advanced resources. In writing this book, I have attempted to produce a concise textbook that meets ...
Theories of the constitution of gases in the early nineteenth century
... Chemists showed a utilitarian attitude towards hypotheses and their scepticism seems to have been reserved for those devised merely to explain the behaviour of gases without helping the general progress of chemistry. Dalton’s theory, supported by the laws of constant, multiple and equivalent proport ...
... Chemists showed a utilitarian attitude towards hypotheses and their scepticism seems to have been reserved for those devised merely to explain the behaviour of gases without helping the general progress of chemistry. Dalton’s theory, supported by the laws of constant, multiple and equivalent proport ...
theodore l. brown h. eugene lemay, jr. bruce e. bursten catherine j
... system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or likewise. To obtain permission(s) to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1900 E. Lake Ave., Glenview, IL 60025. Many o ...
... system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or likewise. To obtain permission(s) to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1900 E. Lake Ave., Glenview, IL 60025. Many o ...
Personal Tutor - Macmillan Learning
... components: precision and accuracy. Precision refers to how closely measurements of the same quantity agree. A high-precision measurement is one that produces very nearly the same result each time it is measured. Accuracy is how well measurements agree with the accepted or true value. It is possible ...
... components: precision and accuracy. Precision refers to how closely measurements of the same quantity agree. A high-precision measurement is one that produces very nearly the same result each time it is measured. Accuracy is how well measurements agree with the accepted or true value. It is possible ...
Word - icho39.chem.msu.ru
... Problem 1. ON THE BORDERS OF THE PERIODIC SYSTEM 1. In 1875 the French chemist Paul-Emile Lecoq de Boisbaudran studied the spectra of zinc ore and discovered the traces of a new element, which he called “gallium” from the Latin word "Gallia" meaning "France" and perhaps also from the Latin word "gal ...
... Problem 1. ON THE BORDERS OF THE PERIODIC SYSTEM 1. In 1875 the French chemist Paul-Emile Lecoq de Boisbaudran studied the spectra of zinc ore and discovered the traces of a new element, which he called “gallium” from the Latin word "Gallia" meaning "France" and perhaps also from the Latin word "gal ...
Gas Laws
... 6. Why is hydrogen bonding only possible with hydrogen? Hydrogen is the only element that has an exposed proton when an electron is lost. The exposure of the proton and the fact that the other element that the hydrogen in bonded to has a very high electron affinity, the compound ends up having a ver ...
... 6. Why is hydrogen bonding only possible with hydrogen? Hydrogen is the only element that has an exposed proton when an electron is lost. The exposure of the proton and the fact that the other element that the hydrogen in bonded to has a very high electron affinity, the compound ends up having a ver ...
Chapter 4
... Since the reaction is occurring in basic solution, the hydrogen ions (H+) must be neutralized by adding equal numbers of OH- ions to both sides of the equations. 8 OH- + 2 H2O + N2H4 → 2 NO + 8 H+ + 8 e- + 8 OH- ...
... Since the reaction is occurring in basic solution, the hydrogen ions (H+) must be neutralized by adding equal numbers of OH- ions to both sides of the equations. 8 OH- + 2 H2O + N2H4 → 2 NO + 8 H+ + 8 e- + 8 OH- ...
Gas Laws
... 6. Why is hydrogen bonding only possible with hydrogen? Hydrogen is the only element that has an exposed proton when an electron is lost. The exposure of the proton and the fact that the other element that the hydrogen in bonded to has a very high electron affinity, the compound ends up having a ver ...
... 6. Why is hydrogen bonding only possible with hydrogen? Hydrogen is the only element that has an exposed proton when an electron is lost. The exposure of the proton and the fact that the other element that the hydrogen in bonded to has a very high electron affinity, the compound ends up having a ver ...
CHEMICAL REACTIONS
... – Chemical reactions occur when old bonds between atoms are broken and new bonds between atoms are formed – Chemical reactions involve chemical changes in matter. • New substances with new properties are created (products). ...
... – Chemical reactions occur when old bonds between atoms are broken and new bonds between atoms are formed – Chemical reactions involve chemical changes in matter. • New substances with new properties are created (products). ...