ESO - ENCIGA
... order to be able to predict its behaviour and understand its history. Science is based on systematic experimentation and on observation of natural phenomena to discover facts about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific ...
... order to be able to predict its behaviour and understand its history. Science is based on systematic experimentation and on observation of natural phenomena to discover facts about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific ...
Contents
... to a class of compounds referred to as coordination compounds, metal complexes, or just complexes. These compounds contain a central atom or ion, usually a metal, surrounded by several ions or molecules. The complex tends to retain its identity even in solution, although partial dissociation may occ ...
... to a class of compounds referred to as coordination compounds, metal complexes, or just complexes. These compounds contain a central atom or ion, usually a metal, surrounded by several ions or molecules. The complex tends to retain its identity even in solution, although partial dissociation may occ ...
Chem 2A Final Review
... 9. The answer closest to the number of grams of NaH2PO4 needed to react with 38.74 mL of 0.275 M NaOH, according to the following balanced equation is: (NaH2PO4 = 119.98 g/mol) NaH2PO4 (s) + 2NaOH (aq) Na3POH4 (aq) + 3H2O ...
... 9. The answer closest to the number of grams of NaH2PO4 needed to react with 38.74 mL of 0.275 M NaOH, according to the following balanced equation is: (NaH2PO4 = 119.98 g/mol) NaH2PO4 (s) + 2NaOH (aq) Na3POH4 (aq) + 3H2O ...
Organic Chemistry Organic Chemistry
... When a C atom is single-bonded to another C atom, the bond is a strong covalent bond that is difficult to break. Thus, the sites in organic molecules that contain C2C bonds are not reactive. However, double or triple bonds between C atoms are more reactive. The second and third bonds formed in a mul ...
... When a C atom is single-bonded to another C atom, the bond is a strong covalent bond that is difficult to break. Thus, the sites in organic molecules that contain C2C bonds are not reactive. However, double or triple bonds between C atoms are more reactive. The second and third bonds formed in a mul ...
elements of chemistry unit
... Example 5. Aluminum and oxygen react to form aluminum oxide: 4 Al(s) + 3 O2(g) 2 Al2O3(s) 5a. Predict the before and after oxidation numbers for aluminum and oxygen. A. For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation n ...
... Example 5. Aluminum and oxygen react to form aluminum oxide: 4 Al(s) + 3 O2(g) 2 Al2O3(s) 5a. Predict the before and after oxidation numbers for aluminum and oxygen. A. For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation n ...
Diversity-oriented synthesis - David Spring
... space analysis is used. Each molecule, as a function of its associated chemical descriptors,21–24 resides at a discrete point in chemical space.25 Natural products and currently available compound libraries occupy only a small proportion of bioactive chemical space.8,25 Therefore, there may be value ...
... space analysis is used. Each molecule, as a function of its associated chemical descriptors,21–24 resides at a discrete point in chemical space.25 Natural products and currently available compound libraries occupy only a small proportion of bioactive chemical space.8,25 Therefore, there may be value ...
Gas phase chemistry of neutral metal clusters
... Catalytic properties of a material (activity, selectivity, and stability) are, in general, determined by chemical (electronic) properties of surface atoms/molecules [11,12]. On the basis of the concept that a catalytic reaction occurs at specific locally active sites [6,13,14], gas phase metal, metal ...
... Catalytic properties of a material (activity, selectivity, and stability) are, in general, determined by chemical (electronic) properties of surface atoms/molecules [11,12]. On the basis of the concept that a catalytic reaction occurs at specific locally active sites [6,13,14], gas phase metal, metal ...
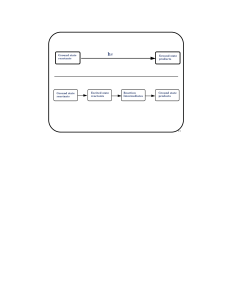
Ground state reactants Ground state products Ground state
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
«Классы и номенклатура неорганических соединений»
... D.the number of protons and neutrons in the nuclear of atom E. the mass of atomic nuclear. 15. What is the correct electron configuration for ion Fe3+, if the electron configuration for iron atom is [Ar]3d6 4s2 A. * [Ar] 3d5 4s0 B. [Ar] 3d5 4s1 4p1 C. [Ar] 3d3 4s2 D. [Ar] 3d3 4s1 4p1 E. [Ar] 3d4 4s1 ...
... D.the number of protons and neutrons in the nuclear of atom E. the mass of atomic nuclear. 15. What is the correct electron configuration for ion Fe3+, if the electron configuration for iron atom is [Ar]3d6 4s2 A. * [Ar] 3d5 4s0 B. [Ar] 3d5 4s1 4p1 C. [Ar] 3d3 4s2 D. [Ar] 3d3 4s1 4p1 E. [Ar] 3d4 4s1 ...
Synthesis, identification and thermal decomposition of double
... of the double sul®tes are also in agreement with the Xray powder diffraction patterns. On the other hand, the X-ray diffraction data obtained from the ®rst and second stages did not allow to identify the intermediary compounds due to their high complexity. In the present work, it was dif®cult to dis ...
... of the double sul®tes are also in agreement with the Xray powder diffraction patterns. On the other hand, the X-ray diffraction data obtained from the ®rst and second stages did not allow to identify the intermediary compounds due to their high complexity. In the present work, it was dif®cult to dis ...
- Angelo State University
... Chemical Reactions and Chemical Equations • A chemical reaction occurs when atoms of different elements combine and create a new chemical compound, with properties which may be completely unlike those of its constituent elements. • A chemical reaction is written in a standard format called a chemica ...
... Chemical Reactions and Chemical Equations • A chemical reaction occurs when atoms of different elements combine and create a new chemical compound, with properties which may be completely unlike those of its constituent elements. • A chemical reaction is written in a standard format called a chemica ...
elements of chemistry unit
... Rule 1. As shown earlier, the oxidation number of atoms in a pure element is defined as zero: C(0) Fe(0) H2(0) Rule 2. A single atom is assigned an oxidation number equal to its electrical charge. For metals, electrical charges are assigned to the metal’s number of valence electrons. Examples are Na ...
... Rule 1. As shown earlier, the oxidation number of atoms in a pure element is defined as zero: C(0) Fe(0) H2(0) Rule 2. A single atom is assigned an oxidation number equal to its electrical charge. For metals, electrical charges are assigned to the metal’s number of valence electrons. Examples are Na ...
Atoms, Molecules and Ions - Wantagh Union Free School District
... Paris, and although Lavoisier's father wanted him to be a lawyer, Lavoisier was fascinated by science. From the beginning of his scientific career, Lavoisier recognized the importance of accurate measurements. He wrote the first modern chemistry (1789) textbook so that it is not surprising that Lavo ...
... Paris, and although Lavoisier's father wanted him to be a lawyer, Lavoisier was fascinated by science. From the beginning of his scientific career, Lavoisier recognized the importance of accurate measurements. He wrote the first modern chemistry (1789) textbook so that it is not surprising that Lavo ...
CHM 423 Coordination Chemistry
... 5.0 Summary 6.0 Tutor-Marked Assignment (TMA) 7.0 References/Further Readings ...
... 5.0 Summary 6.0 Tutor-Marked Assignment (TMA) 7.0 References/Further Readings ...
Redox
... REDOX EQUATIONS Constructing Half –Equations The half-equation shows either the oxidation or the reduction step of a redox change. In a half-equation: ...
... REDOX EQUATIONS Constructing Half –Equations The half-equation shows either the oxidation or the reduction step of a redox change. In a half-equation: ...
The decomposition of hydrogen peroxide to form water and oxygen
... Section II Directions: Questions 1 through 3 are long constructed response questions that should require about 20 minutes to answer. Questions 4 through 7 are short constructed response questions that should require about 7 minutes each to answer. Read each question carefully and write your response ...
... Section II Directions: Questions 1 through 3 are long constructed response questions that should require about 20 minutes to answer. Questions 4 through 7 are short constructed response questions that should require about 7 minutes each to answer. Read each question carefully and write your response ...
some basic concepts of chemistry
... molecular formula for a compound from the given experimental data; • perform the stoichiometric calculations. ...
... molecular formula for a compound from the given experimental data; • perform the stoichiometric calculations. ...
Enthalpy change
... to tell if standard conditions are used we modify the symbol for ∆Η . Enthalpy Change ...
... to tell if standard conditions are used we modify the symbol for ∆Η . Enthalpy Change ...
IChO 2012
... (1875–1946) early in the 20 th century. That is, acids are electron-pair acceptors, whereas bases are electron-pair donors. There are thousands of molecules that can be classified as Lewis acids or bases, and hundreds of studies of the quantitative aspects of Lewis acid-base chemistry were carried o ...
... (1875–1946) early in the 20 th century. That is, acids are electron-pair acceptors, whereas bases are electron-pair donors. There are thousands of molecules that can be classified as Lewis acids or bases, and hundreds of studies of the quantitative aspects of Lewis acid-base chemistry were carried o ...
4) What is the term for the procedure of collecting data and recording
... A sample of rose gold is: 12.0 grams gold, 5.0 grams silver, and 7.0 grams copper by mass. What is the percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% ...
... A sample of rose gold is: 12.0 grams gold, 5.0 grams silver, and 7.0 grams copper by mass. What is the percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% ...