Stoichiometric Conversions
... Predict the products and write a balanced equation for the following: ...
... Predict the products and write a balanced equation for the following: ...
CfE Advanced Higher Chemistry
... up as dark lines on a continuous spectrum and is called an atomic absorption spectrum, see Figure 1.4 (c). This also provides a pattern that can often be used in identification. In both techniques some lines normally occur in the visible region (400-700 nm) but some applications use the ultraviolet ...
... up as dark lines on a continuous spectrum and is called an atomic absorption spectrum, see Figure 1.4 (c). This also provides a pattern that can often be used in identification. In both techniques some lines normally occur in the visible region (400-700 nm) but some applications use the ultraviolet ...
CHE-310 Organic Chemistry I_
... One way to prepare would be to redo all of your homework as if it were an exam. Then, pay particular attention to those problems that you cannot do or do not understand. If you have questions about any of the assigned material you should seek help immediately. - be able to define important terms. - ...
... One way to prepare would be to redo all of your homework as if it were an exam. Then, pay particular attention to those problems that you cannot do or do not understand. If you have questions about any of the assigned material you should seek help immediately. - be able to define important terms. - ...
The d- and f- Block Element Block Elements The d- and f
... new electron enters a d orbital each time the nuclear charge increases by unity. It may be recalled that the shielding effect of a d electron is not that effective, hence the net electrostatic attraction between the nuclear charge and the outermost electron increases and the ionic radius decreases. ...
... new electron enters a d orbital each time the nuclear charge increases by unity. It may be recalled that the shielding effect of a d electron is not that effective, hence the net electrostatic attraction between the nuclear charge and the outermost electron increases and the ionic radius decreases. ...
Practice Exam I FR Answers and Explanations
... (1pt) PF3 has a trigonal pyramidal shape. The one unshared electron pair on the central atom pulls with greater force on phosphorous than do the shared pairs of electrons with fluorine. Because of this unequal pull on the central atom, the molecule has a net dipole moment and is polar. PF5 is a trig ...
... (1pt) PF3 has a trigonal pyramidal shape. The one unshared electron pair on the central atom pulls with greater force on phosphorous than do the shared pairs of electrons with fluorine. Because of this unequal pull on the central atom, the molecule has a net dipole moment and is polar. PF5 is a trig ...
TOPIC 7. CHEMICAL CALCULATIONS I
... CHEMICAL CALCULATIONS I - atomic and formula weights. Atomic structure revisited. In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually no neutrons in its nucleus with one electron orbiting outside that nucleus, through to very large atoms such ...
... CHEMICAL CALCULATIONS I - atomic and formula weights. Atomic structure revisited. In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually no neutrons in its nucleus with one electron orbiting outside that nucleus, through to very large atoms such ...
Year 11 C2 Mock Exam Revision Questions
... Phosphorus and fluorine form a covalent compound, phosphorus trifluoride. Complete the sentences below which are about this compound. Phosphorus trifluoride is made up of phosphorus and fluorine ................................ These are joined together by sharing pairs of .......................... ...
... Phosphorus and fluorine form a covalent compound, phosphorus trifluoride. Complete the sentences below which are about this compound. Phosphorus trifluoride is made up of phosphorus and fluorine ................................ These are joined together by sharing pairs of .......................... ...
TEKS 8 - UNT College of Education
... A chemical reaction, also called a chemical change, is material changing from a beginning mass to a resulting substance. The process involves one or more reactants yielding one or more products different from the reactants. The characteristic of a chemical reaction is that new material or materials ...
... A chemical reaction, also called a chemical change, is material changing from a beginning mass to a resulting substance. The process involves one or more reactants yielding one or more products different from the reactants. The characteristic of a chemical reaction is that new material or materials ...
Chemical Reactions and Equations - 2012 Book Archive
... discussed how the products of a chemical reaction can be predicted. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be. A single-replacement reaction6 is a chemical reaction in which one element is substituted for an ...
... discussed how the products of a chemical reaction can be predicted. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be. A single-replacement reaction6 is a chemical reaction in which one element is substituted for an ...
Oxidation-Reduction Reactions
... Many elements simply combine with oxygen to form the oxide of that element. Heating magnesium in air allows it to combine with oxygen to form magnesium oxide. 2 Mg(s) + O2 (g) → 2MgO(s) Many compounds react with oxygen as well, often in very exothermic processes that are generally referred to as com ...
... Many elements simply combine with oxygen to form the oxide of that element. Heating magnesium in air allows it to combine with oxygen to form magnesium oxide. 2 Mg(s) + O2 (g) → 2MgO(s) Many compounds react with oxygen as well, often in very exothermic processes that are generally referred to as com ...
Brilliant Preparatory Section, Sitamarhi
... viii. All the reactants and products should be written as molecules including the elements like hydrogen, oxygen, nitrogen, fluorine chlorine, bromine and iodine as H2, O2, N2, F2, Cl2, Br2 and I2. ...
... viii. All the reactants and products should be written as molecules including the elements like hydrogen, oxygen, nitrogen, fluorine chlorine, bromine and iodine as H2, O2, N2, F2, Cl2, Br2 and I2. ...
Experimental and Computational Evidence of Metal‑O2 Activation
... catalytic reactions.36−47 These probes are particularly useful when combined with DFT calculations, which can provide information regarding transition states that would not otherwise be accessible.11,48−55 The 18O KIE is defined by the ratio of rate constants, kcat/KM(16,16O2) to kcat/KM(16,18O2). Th ...
... catalytic reactions.36−47 These probes are particularly useful when combined with DFT calculations, which can provide information regarding transition states that would not otherwise be accessible.11,48−55 The 18O KIE is defined by the ratio of rate constants, kcat/KM(16,16O2) to kcat/KM(16,18O2). Th ...
Stoichiometry of Chemical Reactions
... Solid-fuel rockets are a central feature in the world’s space exploration programs, including the new Space Launch System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefull ...
... Solid-fuel rockets are a central feature in the world’s space exploration programs, including the new Space Launch System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefull ...
Unit_4_Notes_
... found using t½ = 0.693 / k o Half-life of a second-order reaction can be found using t½ = 1 / k[A]0 14.5 Temperature and Rate Temperature causes the rate of a chemical reaction to increase because the temperature increase causes the rate constant to increase The collision model, which is based o ...
... found using t½ = 0.693 / k o Half-life of a second-order reaction can be found using t½ = 1 / k[A]0 14.5 Temperature and Rate Temperature causes the rate of a chemical reaction to increase because the temperature increase causes the rate constant to increase The collision model, which is based o ...
Document
... governing the conversion of substrate to product following the initial collision of substrate with enzyme. These include conformational changes in the enzymeSubstrate complex, chemical reactions (including the formation and breakdown of intermediates), and conformational changes that limit the rate ...
... governing the conversion of substrate to product following the initial collision of substrate with enzyme. These include conformational changes in the enzymeSubstrate complex, chemical reactions (including the formation and breakdown of intermediates), and conformational changes that limit the rate ...
The Mole
... an amount equal to the atomic mass of the element expressed in grams. • Example: for Neon, Atomic Mass = 20.18 amu Molar Mass = 20.18 g ...
... an amount equal to the atomic mass of the element expressed in grams. • Example: for Neon, Atomic Mass = 20.18 amu Molar Mass = 20.18 g ...
PRACTICE EXERCISE - Needham.K12.ma.us
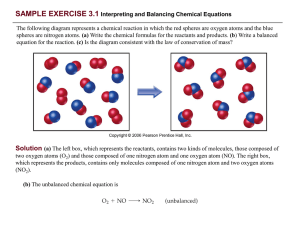
... one O2 for each two NO as required by the balanced equation. The right box (products) contains eight NO 2 molecules. The number of NO2 molecules on the right equals the number of NO molecules on the left as the balanced equation requires. Counting the atoms, we find eight N atoms in the eight NO mol ...
... one O2 for each two NO as required by the balanced equation. The right box (products) contains eight NO 2 molecules. The number of NO2 molecules on the right equals the number of NO molecules on the left as the balanced equation requires. Counting the atoms, we find eight N atoms in the eight NO mol ...
Photocatalysis on TiOn Surfaces: Principles, Mechanisms, and
... Here a and p correspond to the only two spin configurations, spin-up ( a )and spin-down CP). The electron spin functions maintain their orthogonality and normalization properties even when the electrons under consideration are described by different spatial wave functions. Using these properties, we ...
... Here a and p correspond to the only two spin configurations, spin-up ( a )and spin-down CP). The electron spin functions maintain their orthogonality and normalization properties even when the electrons under consideration are described by different spatial wave functions. Using these properties, we ...
here
... alone. We need to ask specific questions and conduct experiments to find answers. Scientists develop laws through experimentation and observation. After experimenting on or observing some facet of nature, they formulate a hypothesis to explain their observations. A hypothesis is no more than an educ ...
... alone. We need to ask specific questions and conduct experiments to find answers. Scientists develop laws through experimentation and observation. After experimenting on or observing some facet of nature, they formulate a hypothesis to explain their observations. A hypothesis is no more than an educ ...
04 Reactions in Aqueous Solution
... • Spectator ions--not involved in overall reaction. 3. Net ionic equation • Shows only the species that take part in the reaction. ...
... • Spectator ions--not involved in overall reaction. 3. Net ionic equation • Shows only the species that take part in the reaction. ...
The Oxidation States of Tin
... of the experiment following the oxidation of the metal compound by the iodine. This is significant because it shows that the reaction was proceeding in the predicted way. It also helps to support the fact that this was the iodine-metal redox reaction that was occurring. This was due to the fact that ...
... of the experiment following the oxidation of the metal compound by the iodine. This is significant because it shows that the reaction was proceeding in the predicted way. It also helps to support the fact that this was the iodine-metal redox reaction that was occurring. This was due to the fact that ...
CHAPTER 3 STOICHIOMETRY:
... For molecular compounds: The formula weight is also called the molecular weight (MW). For ionic compounds: No separated molecules so it called specifically the formula weight (FW). For Elements: In case of isolated atoms is called atomic weight (AW). FW of NaCl = 23 amu + 35.5 amu = 58.5 amu ...
... For molecular compounds: The formula weight is also called the molecular weight (MW). For ionic compounds: No separated molecules so it called specifically the formula weight (FW). For Elements: In case of isolated atoms is called atomic weight (AW). FW of NaCl = 23 amu + 35.5 amu = 58.5 amu ...