Chemical Kinetics
... Integrated first-order rate law is ln[A] = kt + ln[A]o 1. The equation shows how [A] depends on time. 2. Is in the form y=mx + b, where a plot of y vs. x is a straight line with slope m and ...
... Integrated first-order rate law is ln[A] = kt + ln[A]o 1. The equation shows how [A] depends on time. 2. Is in the form y=mx + b, where a plot of y vs. x is a straight line with slope m and ...
Safety Quiz - WordPress.com
... nonmetals: nonlustrous (many are colored), may be solids, liquids, or gases, poor conductors of heat and electricity, form anions, solid nonmetals are brittle, form acidic oxides. (0.5 points each for a maximum of 2 points) ...
... nonmetals: nonlustrous (many are colored), may be solids, liquids, or gases, poor conductors of heat and electricity, form anions, solid nonmetals are brittle, form acidic oxides. (0.5 points each for a maximum of 2 points) ...
Unit 12: Electrochemistry
... Redox Reactions Objective: What steps do we take to balance Redox Reactions? Balancing Redox Reactions: Previous examples have shown how the spectator ions in a redox reaction may be ignored during redox reactions. We can therefore eliminate any spectator ions from the balancing of half-reactions. ...
... Redox Reactions Objective: What steps do we take to balance Redox Reactions? Balancing Redox Reactions: Previous examples have shown how the spectator ions in a redox reaction may be ignored during redox reactions. We can therefore eliminate any spectator ions from the balancing of half-reactions. ...
LaBrake, Fundamentals Diagnostic Questions
... 45. How many moles of glucose are there in 2.4088 × 1024 molecules of glucose? a) 4 moles b) 2 moles c) 6.0221 × 1023 moles d) 1.4506 × 1048 moles e) insufficient information to answer 46. Calculate the molar mass of copper (II) nitrate, Cu(NO3)2. a) 187.57 g·mol-1 b) 93.56 g·mol-1 c) 125.56 g·mol- ...
... 45. How many moles of glucose are there in 2.4088 × 1024 molecules of glucose? a) 4 moles b) 2 moles c) 6.0221 × 1023 moles d) 1.4506 × 1048 moles e) insufficient information to answer 46. Calculate the molar mass of copper (II) nitrate, Cu(NO3)2. a) 187.57 g·mol-1 b) 93.56 g·mol-1 c) 125.56 g·mol- ...
Chemistry booklet
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
Hydrocarbons and Fuels - Deans Community High School
... Each OH group in the glycerol can combine chemically with one carboxylic fatty acid molecule. The resulting molecules are fats and oils. Question: What is the name of this reaction? They are described as triglycerides. (tri esters) The hydrocarbon chain in each can be from 4 to 24 C’s long. The C’s ...
... Each OH group in the glycerol can combine chemically with one carboxylic fatty acid molecule. The resulting molecules are fats and oils. Question: What is the name of this reaction? They are described as triglycerides. (tri esters) The hydrocarbon chain in each can be from 4 to 24 C’s long. The C’s ...
Development of Novel Catalytic Asymmetric Reactions using
... can significantly enhance the reactivity of imines. Therefore, encouraged by the cooperative action presented in Scheme 9, we also investigated a Mannich-type reaction employing imine, which exhibits high affinity to protic acid, as the electrophilic agent. We found that the aqua complex 1 was effec ...
... can significantly enhance the reactivity of imines. Therefore, encouraged by the cooperative action presented in Scheme 9, we also investigated a Mannich-type reaction employing imine, which exhibits high affinity to protic acid, as the electrophilic agent. We found that the aqua complex 1 was effec ...
IChO_Comp_Prob_Answ 1997
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
29th INTERNATIONAL CHEMISTRY OLYMPIAD PREPARATORY
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
Chemistry 1250 - Sp17 Solutions for Midterm 1
... of water to determine density. Remember, D = m/V, which can be written as m = D*V (m = D*V + 0). Graphs were made of mass vs. volume (y vs. x) and the data was fit to a “best-fit” line. A best-fit line averages out the error in the data. The slope of the line would be equal to the density of the liq ...
... of water to determine density. Remember, D = m/V, which can be written as m = D*V (m = D*V + 0). Graphs were made of mass vs. volume (y vs. x) and the data was fit to a “best-fit” line. A best-fit line averages out the error in the data. The slope of the line would be equal to the density of the liq ...
Covalent Bonding and Molecular Geometry - Dr. Vernon-
... Covalent Bonding and Molecular Geometry Directions: Determine the number of electrons and electron pairs. Use dashes to draw the structural formula for the compound in the left side of the box. Later, you’ll redraw with proper bonds angles on the right side and write the name describing the molecule ...
... Covalent Bonding and Molecular Geometry Directions: Determine the number of electrons and electron pairs. Use dashes to draw the structural formula for the compound in the left side of the box. Later, you’ll redraw with proper bonds angles on the right side and write the name describing the molecule ...
AP Chemistry Notes and Worksheets 2014
... covalent bonds-created when two or more nonmetals share electrons molecule- atoms held together by covalent bonds ions- charged particles formed by the loss or gain of electrons ionic bonds- compounds created when one atom loses an electron and another gains it; are held together by electros ...
... covalent bonds-created when two or more nonmetals share electrons molecule- atoms held together by covalent bonds ions- charged particles formed by the loss or gain of electrons ionic bonds- compounds created when one atom loses an electron and another gains it; are held together by electros ...
Iron Oxyhydroxide Aerogels and Xerogels by Hydrolysis of FeCl3 ∙ 6
... can be applied for removal of excess water with preservation of the high porosity.[5,6] ...
... can be applied for removal of excess water with preservation of the high porosity.[5,6] ...
Minimum electrophilicity principle in Lewis acid–base complexes of
... Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes are formed by stronger acids. Therefore, according to the MHP and MEP, for each set of complexes which are formed for a given base and different acids, the ...
... Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes are formed by stronger acids. Therefore, according to the MHP and MEP, for each set of complexes which are formed for a given base and different acids, the ...
mole concept a
... In the previous section we have learned that a mole of a substance is that amount which contains as many elementary entities as there are atoms in exactly 0.012 kilogram or 12 gram of the carbon-12 isotope. This definition gives us a method by which we can find out the amount of a substance (in mole ...
... In the previous section we have learned that a mole of a substance is that amount which contains as many elementary entities as there are atoms in exactly 0.012 kilogram or 12 gram of the carbon-12 isotope. This definition gives us a method by which we can find out the amount of a substance (in mole ...
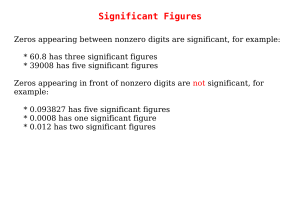
Significant Figures
... * 35.00 has four significant figures * 8,000.000000 has ten significant figures Zeros at the end of a number without a decimal point may or may not be significant, and are therefore ambiguous, for example: * 1,000 could have between one and four significant figures. This ambiguity could be resolved ...
... * 35.00 has four significant figures * 8,000.000000 has ten significant figures Zeros at the end of a number without a decimal point may or may not be significant, and are therefore ambiguous, for example: * 1,000 could have between one and four significant figures. This ambiguity could be resolved ...
Learning Outcomes
... (b) describe, with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shells (energy levels) (Knowledge of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ...
... (b) describe, with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shells (energy levels) (Knowledge of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ...
Stoichiometry of Ozonation of Environmentally
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
The Wizard Test Maker
... An experiment is set up to determine the molecular mass of a water-soluble, nonvolatile, non-electrolyte. The equipment listed above is availeable to use. No other equipment is available. (a) Briefly list the steps needed to carry out this experiment. (b) What experimental data needs to be collected ...
... An experiment is set up to determine the molecular mass of a water-soluble, nonvolatile, non-electrolyte. The equipment listed above is availeable to use. No other equipment is available. (a) Briefly list the steps needed to carry out this experiment. (b) What experimental data needs to be collected ...
TOPIC 7. CHEMICAL CALCULATIONS I
... CHEMICAL CALCULATIONS I - atomic and formula weights. Atomic structure revisited. In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually no neutrons in its nucleus with one electron orbiting outside that nucleus, through to very large atoms such ...
... CHEMICAL CALCULATIONS I - atomic and formula weights. Atomic structure revisited. In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually no neutrons in its nucleus with one electron orbiting outside that nucleus, through to very large atoms such ...