Challenge Problems
... ne of the first somewhat successful attempts to arrange the elements in a systematic way was made by the German chemist Johann Wolfgang Döbereiner (1780–1849). In 1816, Döbereiner noticed that the then accepted atomic mass of strontium (50) was midway between the atomic masses of calcium (27.5) and ...
... ne of the first somewhat successful attempts to arrange the elements in a systematic way was made by the German chemist Johann Wolfgang Döbereiner (1780–1849). In 1816, Döbereiner noticed that the then accepted atomic mass of strontium (50) was midway between the atomic masses of calcium (27.5) and ...
Chem 1B Fa2015 FinalExam Review
... (b) If concentrated nitric acid contains 70.0% (by mass) of HNO3 and the solution has a density of 1.48 g/mL, what is the molar concentration of HNO3 in concentrated nitric acid? (c) How many kilograms of HNO3 are present in 1.o0 gallon (3.785 L) of concentrated nitric acid? (d) How many kilograms o ...
... (b) If concentrated nitric acid contains 70.0% (by mass) of HNO3 and the solution has a density of 1.48 g/mL, what is the molar concentration of HNO3 in concentrated nitric acid? (c) How many kilograms of HNO3 are present in 1.o0 gallon (3.785 L) of concentrated nitric acid? (d) How many kilograms o ...
Review Unit: Chemistry Review
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
Measuring Rates
... The integrated rate law for a chemical reaction expresses how the concentration of a relevant reacting species changes as a function of time. Thus, it can be used to predict the time it will take for a reactant or product to reach a given concentration, or to predict such concentration at a selected ...
... The integrated rate law for a chemical reaction expresses how the concentration of a relevant reacting species changes as a function of time. Thus, it can be used to predict the time it will take for a reactant or product to reach a given concentration, or to predict such concentration at a selected ...
Table of Contents
... undergoes. _____________________ properties are characteristics that a sample of matter exhibits without any change in its identity. This property can be observed and measured without _____________________ the substance. Examples of the physical properties of a chunk of matter include its: 1. ______ ...
... undergoes. _____________________ properties are characteristics that a sample of matter exhibits without any change in its identity. This property can be observed and measured without _____________________ the substance. Examples of the physical properties of a chunk of matter include its: 1. ______ ...
CHEMISTRY OF p-ELEMENTS - Львівський національний
... However, there are not enough electrons for each of the lines shown in the structure to represent an electron pair. Each atom of boron contributes 3 electrons and each atom of hydrogen 1 electron, making a total of 12 electrons, or six pairs for the molecule. Thus there can be a maximum of only six ...
... However, there are not enough electrons for each of the lines shown in the structure to represent an electron pair. Each atom of boron contributes 3 electrons and each atom of hydrogen 1 electron, making a total of 12 electrons, or six pairs for the molecule. Thus there can be a maximum of only six ...
Types of Chemical Reactions
... Two problems 1. Atomic masses do not convert easily to grams 2. They can’t be weighed (they are too small) ...
... Two problems 1. Atomic masses do not convert easily to grams 2. They can’t be weighed (they are too small) ...
Nutrient uptake by protocells: a liposome model system
... (Paula et al., 1996). It follows that transcription of DNA templates by a templatedirected enzyme, such as T7 RNA polymerase, should be possible with the substrates being supplied in the external bulk medium. These are currently underway in our laboratory and will be reported elsewhere. In contrast ...
... (Paula et al., 1996). It follows that transcription of DNA templates by a templatedirected enzyme, such as T7 RNA polymerase, should be possible with the substrates being supplied in the external bulk medium. These are currently underway in our laboratory and will be reported elsewhere. In contrast ...
9.1 REDOX Introduction to Oxidation and Reduction
... oxygen was discovered by Joseph Priestley (17331804) Quickly realized that oxygen forms oxides so the word oxidation was created to describe the addition of oxygen When oxygen is removed “reduction” is used Now oxidation and reduction refer to transfer of ...
... oxygen was discovered by Joseph Priestley (17331804) Quickly realized that oxygen forms oxides so the word oxidation was created to describe the addition of oxygen When oxygen is removed “reduction” is used Now oxidation and reduction refer to transfer of ...
Ionic Liquids Beyond Simple Solvents: Glimpses at the State of the
... tropicity: ions at the beginning of the series are called kosmotropic, ions at the end are chaotropic (cf. text). chemicals. They can easily introduce chirality into a given proAnions that stabilize the native structure of water are called cess, either as a solvent or—much more frequently by reason ...
... tropicity: ions at the beginning of the series are called kosmotropic, ions at the end are chaotropic (cf. text). chemicals. They can easily introduce chirality into a given proAnions that stabilize the native structure of water are called cess, either as a solvent or—much more frequently by reason ...
Atomic Structure
... The more probable formula for the compound formed by atoms x and y both occupying the same period with 2 and 7 electrons respectively is (a) XY ...
... The more probable formula for the compound formed by atoms x and y both occupying the same period with 2 and 7 electrons respectively is (a) XY ...
Topic 7b Redox notes
... CuO is losing oxygen and so is reduced. This happens when heated with hydrogen. Hydrogen has reduced CuO to copper metal and has itself gained oxygen and therefore been oxidised. You could also consider the oxidation states of each substance and would draw the same conclusion. ...
... CuO is losing oxygen and so is reduced. This happens when heated with hydrogen. Hydrogen has reduced CuO to copper metal and has itself gained oxygen and therefore been oxidised. You could also consider the oxidation states of each substance and would draw the same conclusion. ...
Biochemistry Powepoint
... • Water is considered to be a polar molecule due to an uneven distribution of charge. • The electrons in a water molecule are shared unevenly between hydrogen and oxygen. Chapter menu ...
... • Water is considered to be a polar molecule due to an uneven distribution of charge. • The electrons in a water molecule are shared unevenly between hydrogen and oxygen. Chapter menu ...
Chemistry - Set as Home Page
... Chlorine is _________ electronegative than bromine and iodine but _________ electronegative than fluorine. ...
... Chlorine is _________ electronegative than bromine and iodine but _________ electronegative than fluorine. ...
ChemQuest 1 Information: Qualitative vs. Quantitative Critical
... 5. How are compounds different from mixtures? Compounds are formed by a chemical change (i.e. two hydrogen and one oxygen atom bonding to form a water molecule), but mixtures are formed by a physical change (i.e. stirring salt and water together. 6. How are pure substances different from mixtures? P ...
... 5. How are compounds different from mixtures? Compounds are formed by a chemical change (i.e. two hydrogen and one oxygen atom bonding to form a water molecule), but mixtures are formed by a physical change (i.e. stirring salt and water together. 6. How are pure substances different from mixtures? P ...
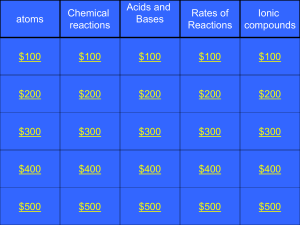
2P chem jeopardy 2011
... When an acid and a base are mixed together, what type of reaction is this and name one product that is produced Category 3: $400: A ...
... When an acid and a base are mixed together, what type of reaction is this and name one product that is produced Category 3: $400: A ...
Redox
... 2H (aq) + 2e → H2 (g) What happened to chloride? It didn’t change (Cl- on both sides of the equation). Ions that don’t change in a reaction are called spectator ions. ...
... 2H (aq) + 2e → H2 (g) What happened to chloride? It didn’t change (Cl- on both sides of the equation). Ions that don’t change in a reaction are called spectator ions. ...
chlorine bromine iodine Halogen Reaction with Iron wool
... oxidized and reduced; for example, chlorine in: Cl2 + H2O > HCl + HOCl. • electronegativity – The power of an atom to withdraw electron density and attract the pair of bonding electrons in a covalent bond. • half equation – An equation describing either the oxidation or reduction in a redox reactio ...
... oxidized and reduced; for example, chlorine in: Cl2 + H2O > HCl + HOCl. • electronegativity – The power of an atom to withdraw electron density and attract the pair of bonding electrons in a covalent bond. • half equation – An equation describing either the oxidation or reduction in a redox reactio ...
St. Xavier`s College – Autonomous Mumbai Syllabus for 3 Semester
... 1.1.4: Covalent bond: Writing Lewis structures, formal charge and Lewis structures, concept of resonance and resonance energy, exceptions to the octet rule, bond enthalpy. 1.1.5: Sidgwick -Powell Theory. 1.1.6: VSEPR concept: Effect of lone pairs, effect of electronegativity, isoelectronic principle ...
... 1.1.4: Covalent bond: Writing Lewis structures, formal charge and Lewis structures, concept of resonance and resonance energy, exceptions to the octet rule, bond enthalpy. 1.1.5: Sidgwick -Powell Theory. 1.1.6: VSEPR concept: Effect of lone pairs, effect of electronegativity, isoelectronic principle ...
Nikolai N. Semenov - Nobel Lecture
... theory of structure. Unfortunately, these rules were in most cases only qualitative; they had many exceptions and were often dependent upon the test conditions. Nowadays, roughly a hundred years since the advent of the theory of structure, questions of reaction velocity are dealt with from an electr ...
... theory of structure. Unfortunately, these rules were in most cases only qualitative; they had many exceptions and were often dependent upon the test conditions. Nowadays, roughly a hundred years since the advent of the theory of structure, questions of reaction velocity are dealt with from an electr ...
Chapter 8 and 9
... From this information you can calculate the amount of carbon and hydrogen in the sample. However since oxygen is in excess you must find oxygen through indirect means (the mass comes from what is not accounted for by carbon and hydrogen, in a sample that only contains CHO). ...
... From this information you can calculate the amount of carbon and hydrogen in the sample. However since oxygen is in excess you must find oxygen through indirect means (the mass comes from what is not accounted for by carbon and hydrogen, in a sample that only contains CHO). ...