Molecules, Moles and Chemical Equations File
... that releases the same amount of energy. Careful examination of the progress of explosive chemical reactions reveals that they accelerate as they proceed. As a result, all of the available explosive is consumed in a very short period of time. As that happens, the energy from the explosion is also re ...
... that releases the same amount of energy. Careful examination of the progress of explosive chemical reactions reveals that they accelerate as they proceed. As a result, all of the available explosive is consumed in a very short period of time. As that happens, the energy from the explosion is also re ...
Contributions to Function in Blue Copper Proteins
... protein system. There are two regions of focus in variable energy photoelectron spectroscopy (VEPES) experiments. One involves ionization of core electrons (X-ray photoelectron spectroscopy, XPS), the energy giving a direct probe of the effective nuclear charge on the atom and the intensity pattern ...
... protein system. There are two regions of focus in variable energy photoelectron spectroscopy (VEPES) experiments. One involves ionization of core electrons (X-ray photoelectron spectroscopy, XPS), the energy giving a direct probe of the effective nuclear charge on the atom and the intensity pattern ...
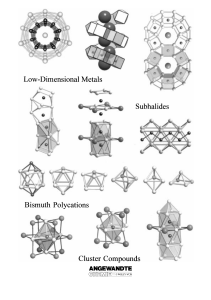
Metalloid Al- and Ga-clusters: a novel dimension in organometallic
... structure analysis.4 The largest cluster of this type is a Pd145 cluster for which—although only two crystals have been obtained so far—the structure could be solved by L. F. Dahl et al.:5 There is a centre of 55 naked Pd atoms surrounded by 90 ligand-bearing Pd atoms. The many synthetic results for ...
... structure analysis.4 The largest cluster of this type is a Pd145 cluster for which—although only two crystals have been obtained so far—the structure could be solved by L. F. Dahl et al.:5 There is a centre of 55 naked Pd atoms surrounded by 90 ligand-bearing Pd atoms. The many synthetic results for ...
AP® Chemistry
... The College Board: Connecting Students to College Success The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded in 1900, the association is composed of more than 5,000 schools, colleges, universities, and other e ...
... The College Board: Connecting Students to College Success The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded in 1900, the association is composed of more than 5,000 schools, colleges, universities, and other e ...
AP® Chemistry
... The College Board: Connecting Students to College Success The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded in 1900, the association is composed of more than 5,000 schools, colleges, universities, and other e ...
... The College Board: Connecting Students to College Success The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded in 1900, the association is composed of more than 5,000 schools, colleges, universities, and other e ...
ch16powerpoint
... Because its 600 bond angles allow poor orbital overlap, its bonds are weak. As a result, it is thermally unstable and rearranges to propene at 10000C via a first-order reaction: • The rate constant is 9.2s-1; (a) What is the half-life of the reaction? (b) How long does it take for the concentration ...
... Because its 600 bond angles allow poor orbital overlap, its bonds are weak. As a result, it is thermally unstable and rearranges to propene at 10000C via a first-order reaction: • The rate constant is 9.2s-1; (a) What is the half-life of the reaction? (b) How long does it take for the concentration ...
Bis2A 06.Appendix A review of Red/Ox reactions
... Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl: H2 (g ) ...
... Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl: H2 (g ) ...
Section 4.9 Oxidation–Reduction Reactions
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
- Career Point Kota
... the P4 molecule where the angle are only 60 & also they have low M.P.” (b) Electron gain enthalpy of halogen are largely negativity it is due to the fact that they have high effective nuclear charge & smallest size among period. Although they contain 7eΘ in valence shell & required one electron to a ...
... the P4 molecule where the angle are only 60 & also they have low M.P.” (b) Electron gain enthalpy of halogen are largely negativity it is due to the fact that they have high effective nuclear charge & smallest size among period. Although they contain 7eΘ in valence shell & required one electron to a ...
Energy Matters - Perth Grammar
... Water molecules formed during the reaction. A precipitate formed during the reaction. The final solution contained equal numbers of H+(aq) and OH− (aq) ions. ...
... Water molecules formed during the reaction. A precipitate formed during the reaction. The final solution contained equal numbers of H+(aq) and OH− (aq) ions. ...
From the Metal to the Molecule
... nonmetal atoms leads, by progressive condensation of the clusters, to increasingly extended metallic regions.[5] Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant pr ...
... nonmetal atoms leads, by progressive condensation of the clusters, to increasingly extended metallic regions.[5] Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant pr ...
L-11 Chemical thermodynamics
... Let us understand it by an example. Imagine a liquid in equlibrium with its vapour in a cylinder closed by a frictionless piston, and placed in a constant temperature bath as shown in figure. 11.3. If the external pressure on the piston is increased by an infinitesimally small amount, the vapours wi ...
... Let us understand it by an example. Imagine a liquid in equlibrium with its vapour in a cylinder closed by a frictionless piston, and placed in a constant temperature bath as shown in figure. 11.3. If the external pressure on the piston is increased by an infinitesimally small amount, the vapours wi ...
M for Moles - Shop
... involving atoms rearrangement. The total number of atoms at the left is always the same as that on the right. Virtually all simple gas molecules are made of two-atom pairs. They are called diatomics. For example, H2, Cl2, N2 and F2. The exceptions are those belong to Group 0. These are called inert ...
... involving atoms rearrangement. The total number of atoms at the left is always the same as that on the right. Virtually all simple gas molecules are made of two-atom pairs. They are called diatomics. For example, H2, Cl2, N2 and F2. The exceptions are those belong to Group 0. These are called inert ...
Organic Chemistry Curriculum Map - Belle Vernon Area School District
... Standard: 3.2.C.A2 – Predict chemical formulas based on the number of valence electrons. Anchor: CHEM.A.1.1 – Identify and describe how observable and measureable properties can be used to classify and describe matter and energy. Eligible Content CHEM.A.1.1.5 – Apply systematic set of rules (IUPAC ...
... Standard: 3.2.C.A2 – Predict chemical formulas based on the number of valence electrons. Anchor: CHEM.A.1.1 – Identify and describe how observable and measureable properties can be used to classify and describe matter and energy. Eligible Content CHEM.A.1.1.5 – Apply systematic set of rules (IUPAC ...
1. What energy changes occur when chemical bonds are formed
... (iv) If the ammonia was produced as a liquid and not as a gas, state and explain the effect this would have on the value of Hο for the reaction. ...
... (iv) If the ammonia was produced as a liquid and not as a gas, state and explain the effect this would have on the value of Hο for the reaction. ...
Measurement and data processing and analysis
... The density should only be given to two significant figures given the uncertainty in the mass and volume values, as discussed in the next section. The uncertainty in the calculated value of the density is 7% (given to one significant figure). This is equal to the sum of the uncertainties in the mass ...
... The density should only be given to two significant figures given the uncertainty in the mass and volume values, as discussed in the next section. The uncertainty in the calculated value of the density is 7% (given to one significant figure). This is equal to the sum of the uncertainties in the mass ...
CFE Higher Chemistry in Society Homework EB
... produce 100cm3 of a 2.5 mol l-1 solution ? 6. Find the mass of:a) sodium chloride in 100 ml of 0.2 moll-1 sodium chloride solution (NaCl) b) glucose in 250ml of 0.05 moll-1 glucose solution (C6H12O6). 7. Find the concentration of:a) 400 ml solution of potassium chloride containing 0.2 moles of potas ...
... produce 100cm3 of a 2.5 mol l-1 solution ? 6. Find the mass of:a) sodium chloride in 100 ml of 0.2 moll-1 sodium chloride solution (NaCl) b) glucose in 250ml of 0.05 moll-1 glucose solution (C6H12O6). 7. Find the concentration of:a) 400 ml solution of potassium chloride containing 0.2 moles of potas ...
Chapter 6 - Sites @ Suffolk University
... : When hydrogen molecules and oxygen molecules react to form water molecules, the atoms form different bonds to make new molecules. The total number of atoms remains the same because the same atoms are present before and after the reaction. But this equation as we have written it is an unbalanced eq ...
... : When hydrogen molecules and oxygen molecules react to form water molecules, the atoms form different bonds to make new molecules. The total number of atoms remains the same because the same atoms are present before and after the reaction. But this equation as we have written it is an unbalanced eq ...
Net ionic equation
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
WA AP Chem gas law IMF MC Set C
... and metals have a high malleability which allows for the mercury to bend slightly under the pressure while maintaining its position, thus decreasing its movement within the manometer. D. The manometer containing mercury, because mercury is denser than water, and therefore requires more pressure to m ...
... and metals have a high malleability which allows for the mercury to bend slightly under the pressure while maintaining its position, thus decreasing its movement within the manometer. D. The manometer containing mercury, because mercury is denser than water, and therefore requires more pressure to m ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... based on its composition. As we saw in Section 2.7, most ionic compounds we encounter in this text are composed of a metal and a nonmetal, whereas most molecular compounds are composed only of nonmetals. Solve: Two compounds fit the criteria for ionic compounds: CaCl 2 and KOH. As Table 2.3 tells us ...
... based on its composition. As we saw in Section 2.7, most ionic compounds we encounter in this text are composed of a metal and a nonmetal, whereas most molecular compounds are composed only of nonmetals. Solve: Two compounds fit the criteria for ionic compounds: CaCl 2 and KOH. As Table 2.3 tells us ...
Power Point over chemistry
... Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chem ...
... Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chem ...