iono-covalent character of the metalааoxygen bonds in oxides
... base is a nucleophile that acts as an electron pair donor. Lewis acid–base interactions play an important role in understanding chemical bonds, reactions and equilibrium; yet, this concept is often inadequate for solid state chemistry. Indeed, in a redox reaction, single-electron transfers are also ...
... base is a nucleophile that acts as an electron pair donor. Lewis acid–base interactions play an important role in understanding chemical bonds, reactions and equilibrium; yet, this concept is often inadequate for solid state chemistry. Indeed, in a redox reaction, single-electron transfers are also ...
Gas-Phase Reactions of Fe (CH2O)+ and Fe (CH2S)+ with Small
... Abstract: The gas-phase reactions of Fe(CH2O)+ and Fe(CH2S)+ with a series of aliphatic alkanes were studied by Fourier transform ion cyclotron resonance (FTICR) mass spectrometry. Like bare Fe+, C-C insertion, particularly terminal C-C insertion, is predominant for the reactions of Fe(CH2O)+, while ...
... Abstract: The gas-phase reactions of Fe(CH2O)+ and Fe(CH2S)+ with a series of aliphatic alkanes were studied by Fourier transform ion cyclotron resonance (FTICR) mass spectrometry. Like bare Fe+, C-C insertion, particularly terminal C-C insertion, is predominant for the reactions of Fe(CH2O)+, while ...
SAMPLE EXERCISE 4.5 Comparing Acid Strengths
... equation and then a net ionic equation for their neutralization reaction. Plan: As Equation 4.12 and the italicized statement that follows it indicate, neutralization reactions form two products, H2O and a salt. We examine the cation of the base and the anion of the acid to determine the composition ...
... equation and then a net ionic equation for their neutralization reaction. Plan: As Equation 4.12 and the italicized statement that follows it indicate, neutralization reactions form two products, H2O and a salt. We examine the cation of the base and the anion of the acid to determine the composition ...
Chapter 3: Ionic and Covalent Compounds Chapter 3: Ionic and
... 102. The sulfite ion contains more oxygen atoms than the sulfate ion. A) True B) False Ans: B Difficulty: Medium 103. The carbonate ion contains the same number of oxygen atoms as the sulfate ion. A) True B) False Ans: B Difficulty: Medium 104. The electron configuration for the sulfide ion is 1s22s ...
... 102. The sulfite ion contains more oxygen atoms than the sulfate ion. A) True B) False Ans: B Difficulty: Medium 103. The carbonate ion contains the same number of oxygen atoms as the sulfate ion. A) True B) False Ans: B Difficulty: Medium 104. The electron configuration for the sulfide ion is 1s22s ...
Document
... equation and then a net ionic equation for their neutralization reaction. Plan: As Equation 4.12 and the italicized statement that follows it indicate, neutralization reactions form two products, H2O and a salt. We examine the cation of the base and the anion of the acid to determine the composition ...
... equation and then a net ionic equation for their neutralization reaction. Plan: As Equation 4.12 and the italicized statement that follows it indicate, neutralization reactions form two products, H2O and a salt. We examine the cation of the base and the anion of the acid to determine the composition ...
Stoichiometry, Lab Basics, Reactions
... ____ 19. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? A) It is equal to 1/3 of the total pressure. B) It depends on ...
... ____ 19. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? A) It is equal to 1/3 of the total pressure. B) It depends on ...
IB Chemistry HL Topic5 Questions 1. Which combination of ionic
... Calculate the standard free energy change at 298 K, GӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use SӨ = –360 J K–1 (this is not the correct value). ...
... Calculate the standard free energy change at 298 K, GӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use SӨ = –360 J K–1 (this is not the correct value). ...
Exames anteriores a 1994
... Upon heating of a mixture of A and fluorine (molar ratio 1:9, high pressure) to 900 °C three compounds (B, C and D) are formed. All three products are crystalline solids with melting points below 150 °C. The fluorine content of C is found to be 36.7% and that of D 46.5% (by weight). When B is treate ...
... Upon heating of a mixture of A and fluorine (molar ratio 1:9, high pressure) to 900 °C three compounds (B, C and D) are formed. All three products are crystalline solids with melting points below 150 °C. The fluorine content of C is found to be 36.7% and that of D 46.5% (by weight). When B is treate ...
chemistry - Textbooks Online
... Eg. Consider the following reaction 2 H2 + O2 → 2H2O In this reaction one molecule of oxygen reacts with two molecules of hydrogen. So it would be desirable to take the molecules of H2 and oxygen in the ratio 2:1, so that the reactants are completely consumed during the reaction. But atoms and mole ...
... Eg. Consider the following reaction 2 H2 + O2 → 2H2O In this reaction one molecule of oxygen reacts with two molecules of hydrogen. So it would be desirable to take the molecules of H2 and oxygen in the ratio 2:1, so that the reactants are completely consumed during the reaction. But atoms and mole ...
Net Ionic Equation Powerpoint Tutorial
... reaction. For example, when AgNO3 solution and KBr solution are mixed together, the Ag+ ions react with the Br- ions to form the insoluble compound: AgBr The net ionic equation is simply: Ag+(aq) + Br-(aq) AgBr(s) Notice how the NO3- and K+ ions are left out of the net ionic equation. Because they ...
... reaction. For example, when AgNO3 solution and KBr solution are mixed together, the Ag+ ions react with the Br- ions to form the insoluble compound: AgBr The net ionic equation is simply: Ag+(aq) + Br-(aq) AgBr(s) Notice how the NO3- and K+ ions are left out of the net ionic equation. Because they ...
Chemistry Unit Outcomes
... station; first aid kit; disposal container for broken glass. Identify the warning labels on various chemicals and household products. Outline the route (or draw a map) that you are to take to exit the school, and the location to which you are to proceed, if the Fire Alarm rings while you are in Clas ...
... station; first aid kit; disposal container for broken glass. Identify the warning labels on various chemicals and household products. Outline the route (or draw a map) that you are to take to exit the school, and the location to which you are to proceed, if the Fire Alarm rings while you are in Clas ...
Ch 4 Student
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
The Mole
... elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. ...
... elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. ...
Atom
... • Fewer electrons (negative) than protons (positive) • Net positive charge • Called a positive ion or cation • One missing electron = charge of +1 • More electrons missing = more positive charge (e.g., ...
... • Fewer electrons (negative) than protons (positive) • Net positive charge • Called a positive ion or cation • One missing electron = charge of +1 • More electrons missing = more positive charge (e.g., ...
Final Study Guide (Semester 2) Answer Key
... ***The first thing you should do when solving this is look at the common ion chart and write down all the ions. It’s much easier than looking them up again for each question. a. Write the balanced molecular equation. Include phase symbols. Ba(NO3)2(aq) + K2SO4(aq) BaSO4(s) + 2KNO3 (aq) Switch the c ...
... ***The first thing you should do when solving this is look at the common ion chart and write down all the ions. It’s much easier than looking them up again for each question. a. Write the balanced molecular equation. Include phase symbols. Ba(NO3)2(aq) + K2SO4(aq) BaSO4(s) + 2KNO3 (aq) Switch the c ...
to view
... electrons occupy anionic vacancies. These sites are called F centers. These electrons absorb energy from the visible region and transmits yellow colour. (ii) In the crystal of FeO, some of the Fe2+ cations are replaced by Fe3+ ions. Three Fe2+ ions are replaced by two Fe3+ ions to make up for the lo ...
... electrons occupy anionic vacancies. These sites are called F centers. These electrons absorb energy from the visible region and transmits yellow colour. (ii) In the crystal of FeO, some of the Fe2+ cations are replaced by Fe3+ ions. Three Fe2+ ions are replaced by two Fe3+ ions to make up for the lo ...
Unit 4, Lesson #3 - Patterson Science
... concentrations of the different species. Once the concentrations have been determined, these values are “plugged into” the expression and Keq is calculated. The wonderful thing about Keq is that it doesn’t matter how much reactant and product you put in the reaction container at the beginning of the ...
... concentrations of the different species. Once the concentrations have been determined, these values are “plugged into” the expression and Keq is calculated. The wonderful thing about Keq is that it doesn’t matter how much reactant and product you put in the reaction container at the beginning of the ...
acid
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
Insertion of SO2 into the Metal−Carbon Bonds of Rhodium and
... been shown to undergo this reaction, and several types of linkages have been observed. A mechanism has been proposed that still holds in the majority of cases.1a While current interest in this reaction has decreased, SO2 remains the subject of numerous studies2 because of its diverse coordination pr ...
... been shown to undergo this reaction, and several types of linkages have been observed. A mechanism has been proposed that still holds in the majority of cases.1a While current interest in this reaction has decreased, SO2 remains the subject of numerous studies2 because of its diverse coordination pr ...
NATIONAL HIGH SCHOOL CHEMISTRY EXAMINATION (1995
... this year is the 100th anniversary of the discovery of X-rays and next year will be the 100th anniversary of radioactivity. Today the words "radiation" and "radioactivity" may bring to mind both positive and negative reactions. Describe the main forms of ionizing radiation, and explain the positive ...
... this year is the 100th anniversary of the discovery of X-rays and next year will be the 100th anniversary of radioactivity. Today the words "radiation" and "radioactivity" may bring to mind both positive and negative reactions. Describe the main forms of ionizing radiation, and explain the positive ...
proline2 - Department of Mathematics and Statistics
... are found close to a planar conformation and can serve as the plane of the proline ring (Chakrabarti & Pal, 2001). Another way to characterize the puckers is by the sign of the torsions, UP (negative 1 and 3, positive 2 and 4) and DOWN (positive 1 and 3, negative 2 and 4). In this study, w ...
... are found close to a planar conformation and can serve as the plane of the proline ring (Chakrabarti & Pal, 2001). Another way to characterize the puckers is by the sign of the torsions, UP (negative 1 and 3, positive 2 and 4) and DOWN (positive 1 and 3, negative 2 and 4). In this study, w ...
Chapter
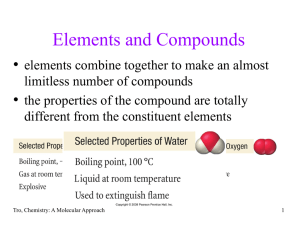
... Formula Mass = 1 molecule of H2O = 2(1.01 amu H) + 16.00 amu O = 18.02 amu • since 1 mole of H2O contains 2 moles of H and 1 mole of O Molar Mass = 1 mole H2O = 2(1.01 g H) + 16.00 g O = 18.02 g so the Molar Mass of H2O is 18.02 g/mole Tro, Chemistry: A Molecular Approach ...
... Formula Mass = 1 molecule of H2O = 2(1.01 amu H) + 16.00 amu O = 18.02 amu • since 1 mole of H2O contains 2 moles of H and 1 mole of O Molar Mass = 1 mole H2O = 2(1.01 g H) + 16.00 g O = 18.02 g so the Molar Mass of H2O is 18.02 g/mole Tro, Chemistry: A Molecular Approach ...