ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 31. What are the three types of radioactive decay you learned about? Write the symbol for each (include all relevant numbers). ...
... 31. What are the three types of radioactive decay you learned about? Write the symbol for each (include all relevant numbers). ...
Atomic number.
... • All living beings are organized. The smallest part of a living organism is the atom. Atoms are the smallest part of an element. An element is a pure substance. • There are 25 different elements necessary to life can be classiffied into: SPONCH (98%) and Trace elements (elements that the body need ...
... • All living beings are organized. The smallest part of a living organism is the atom. Atoms are the smallest part of an element. An element is a pure substance. • There are 25 different elements necessary to life can be classiffied into: SPONCH (98%) and Trace elements (elements that the body need ...
Atomic Structure Test Review 2016
... You may need to check your notes for some definitions. Remember, resources are on ItsLearning. ...
... You may need to check your notes for some definitions. Remember, resources are on ItsLearning. ...
Chapter 5 Notes: The Structure of Matter
... 1. Describe the reaction in words in your head 2. Write the equation using formulas and symbols if it is not already written that way 3. Check for balance with numbers under 4. Add coefficients where needed for balance ...
... 1. Describe the reaction in words in your head 2. Write the equation using formulas and symbols if it is not already written that way 3. Check for balance with numbers under 4. Add coefficients where needed for balance ...
CP Chemistry First Semester Final Exam 1
... 91. The identity of an element is determined by the number of what subatomic particle? 92-96 Tom and Tessa Toro conducted a chemical experiment in which they added varying amounts of magnesium to an excess of HCl. They produced magnesium chloride and hydrogen gas. They captured and measured to hydro ...
... 91. The identity of an element is determined by the number of what subatomic particle? 92-96 Tom and Tessa Toro conducted a chemical experiment in which they added varying amounts of magnesium to an excess of HCl. They produced magnesium chloride and hydrogen gas. They captured and measured to hydro ...
The Chemical Context of Life PPT
... Ionic Bonds: A case study involving greed! • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt ...
... Ionic Bonds: A case study involving greed! • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt ...
The Chemical Context of Life
... Ionic Bonds: A case study involving greed! • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt ...
... Ionic Bonds: A case study involving greed! • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt ...
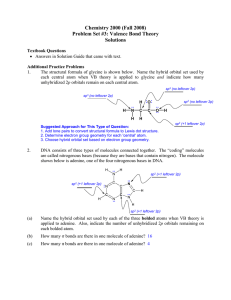
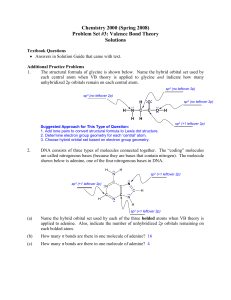
Chemistry 2000 (Fall 2008) Problem Set #3: Valence Bond Theory
... What is the main difference between a hybrid atomic orbital and a molecular orbital? Hybrid atomic orbitals are formed by mixing orbitals on the same atom. ...
... What is the main difference between a hybrid atomic orbital and a molecular orbital? Hybrid atomic orbitals are formed by mixing orbitals on the same atom. ...
Answers - U of L Class Index
... What is the main difference between a hybrid atomic orbital and a molecular orbital? Hybrid atomic orbitals are formed by mixing orbitals on the same atom. ...
... What is the main difference between a hybrid atomic orbital and a molecular orbital? Hybrid atomic orbitals are formed by mixing orbitals on the same atom. ...
Bonding Web Practice Trupia - Trupia
... ____10. Which pair of atoms will share electrons when a bond is formed between them? (1) Ba and I (3) K and Cl (2) Br and Cl (4) Li and I ____11. Covalent bonds are formed when electrons are (1) transferred from one atom to another (2) captured by the nucleus (3) mobile within a metal (4) shared bet ...
... ____10. Which pair of atoms will share electrons when a bond is formed between them? (1) Ba and I (3) K and Cl (2) Br and Cl (4) Li and I ____11. Covalent bonds are formed when electrons are (1) transferred from one atom to another (2) captured by the nucleus (3) mobile within a metal (4) shared bet ...
SEMESTER 1 EXAM Prblms/Short Ans
... 16.Write the formulas for the following binary compounds formed between the following elements: (#1 p. 223) a. Sodium and sulfur _______________________ d. aluminum and nitrogen __________________ Name the binary ionic compound as indicated by the following formulas: (#2 p. 223) b. AgCl ___________ ...
... 16.Write the formulas for the following binary compounds formed between the following elements: (#1 p. 223) a. Sodium and sulfur _______________________ d. aluminum and nitrogen __________________ Name the binary ionic compound as indicated by the following formulas: (#2 p. 223) b. AgCl ___________ ...
Chapter #7 - TeacherWeb
... they are bonded to each other. Place a single covalent bond. 3. Add nonbonding electron pairs to complete the octet. 4. Complete octet around central atom. ...
... they are bonded to each other. Place a single covalent bond. 3. Add nonbonding electron pairs to complete the octet. 4. Complete octet around central atom. ...
Nickel 28 Ni 58.693
... **isotopes will have the same characteristics **Ex: Cl-35 and Cl-37 are chlorine isotopes ...
... **isotopes will have the same characteristics **Ex: Cl-35 and Cl-37 are chlorine isotopes ...
Lesson 1 | Discovering Parts of an Atom
... 1. Many ancient Greek philosophers, such as Aristotle, thought that all matter was made of only four elements—fire, water, air, and ...
... 1. Many ancient Greek philosophers, such as Aristotle, thought that all matter was made of only four elements—fire, water, air, and ...
Chapter 1 - TamAPChemistryHart
... Heating elemental sulfur to high temperatures produces gaseous S2 molecules.: S8(s) 4 S2(g) a) With respect to electronic structure, which element in the second row of the periodic table is most similar to sulfur? b) Use the VSEPR model to predict the S-S-S bond angles in S8 and the hybridization ...
... Heating elemental sulfur to high temperatures produces gaseous S2 molecules.: S8(s) 4 S2(g) a) With respect to electronic structure, which element in the second row of the periodic table is most similar to sulfur? b) Use the VSEPR model to predict the S-S-S bond angles in S8 and the hybridization ...
Molecular Geometry and Chemical Bonding Theory
... Considered a satisfactory method of explaining the electron pair, or covalent bond from a quantum mechanics view. According to this theory, a bond forms between two atoms when the following conditions are met. Two atomic orbitals "overlap" ...
... Considered a satisfactory method of explaining the electron pair, or covalent bond from a quantum mechanics view. According to this theory, a bond forms between two atoms when the following conditions are met. Two atomic orbitals "overlap" ...
Name: Period
... 3. What are the shapes of an s and p orbitals? 4. What is a principal energy level, sublevel and atomic orbital? 5. What is the maximum number in each s, p, d and f orbitals? 6. What types of atomic orbitals are in the 1st, 2nd and 3rd principal energy levels? 7. If the spin of one electron is clock ...
... 3. What are the shapes of an s and p orbitals? 4. What is a principal energy level, sublevel and atomic orbital? 5. What is the maximum number in each s, p, d and f orbitals? 6. What types of atomic orbitals are in the 1st, 2nd and 3rd principal energy levels? 7. If the spin of one electron is clock ...
Elements PPT
... the second can hold eight so it needs two more to be stable, that means that oxygen wants to combine with other elements or itself. ...
... the second can hold eight so it needs two more to be stable, that means that oxygen wants to combine with other elements or itself. ...
Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and
... Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair repulsion (VSEPR) theory and hybrid orbitals (valence bonding). Lewis dot structures give us limite ...
... Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair repulsion (VSEPR) theory and hybrid orbitals (valence bonding). Lewis dot structures give us limite ...