IntroQuantumNuclearp..
... Built on deBroglie and Heisenberg’s ideas...developed more complex wavefunction equation (ψ) model Predicted behavior of e- in space and time – think of it as predicting where and when an e- based on probability* If you map out these likely locations over time, you would see a “cloud” of possible lo ...
... Built on deBroglie and Heisenberg’s ideas...developed more complex wavefunction equation (ψ) model Predicted behavior of e- in space and time – think of it as predicting where and when an e- based on probability* If you map out these likely locations over time, you would see a “cloud” of possible lo ...
7.4 The Quantum-Mechanical Model of the Atom
... – The electron density is highest at the nucleus (density decreases away from the nucleus) – The radial distribution has a maximum slightly away from the nucleus – The orbital size increases with increasing the energy of the orbital (1s < 2s < 3s …) – Higher energy orbitals have several maxima in th ...
... – The electron density is highest at the nucleus (density decreases away from the nucleus) – The radial distribution has a maximum slightly away from the nucleus – The orbital size increases with increasing the energy of the orbital (1s < 2s < 3s …) – Higher energy orbitals have several maxima in th ...
Atomic Structure
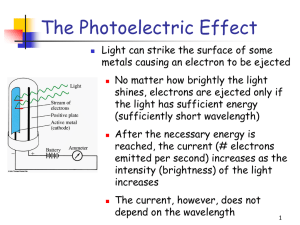
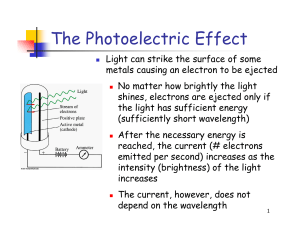
... Light is a wave…right? • Einstein’s interpretation of the photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.) • This meant that light had characteristics of particles! ...
... Light is a wave…right? • Einstein’s interpretation of the photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.) • This meant that light had characteristics of particles! ...
Chemistry (B) Final Exam Study Guide 1
... ____ 54. How are the frequency and wavelength of light related? a. They are inversely proportional to each other. b. Frequency equals wavelength divided by the speed of light. c. Wavelength is determined by dividing frequency by the speed of light. d. They are directly proportional to each other. __ ...
... ____ 54. How are the frequency and wavelength of light related? a. They are inversely proportional to each other. b. Frequency equals wavelength divided by the speed of light. c. Wavelength is determined by dividing frequency by the speed of light. d. They are directly proportional to each other. __ ...
Test #5 Review
... Which is larger, fluorine or bromine? bromine (For the same reason – more energy levels.) Why do elements in the same family behave the same? They all have the same number of valence electrons. ...
... Which is larger, fluorine or bromine? bromine (For the same reason – more energy levels.) Why do elements in the same family behave the same? They all have the same number of valence electrons. ...
ME 533 Lecture 6 Pla..
... and linear polyatomic molecules is somewhat similar to atoms. • quantum number Λ =0,1,2,3 (corresponding Greek symbols Σ, Π, Δ, Φ), describes the absolute value of the component of the total orbital angular momentum along the internuclear axis. • If Λ ≠0, , the states are doubly degenerate because o ...
... and linear polyatomic molecules is somewhat similar to atoms. • quantum number Λ =0,1,2,3 (corresponding Greek symbols Σ, Π, Δ, Φ), describes the absolute value of the component of the total orbital angular momentum along the internuclear axis. • If Λ ≠0, , the states are doubly degenerate because o ...
1s 2s 2p - Solon City Schools
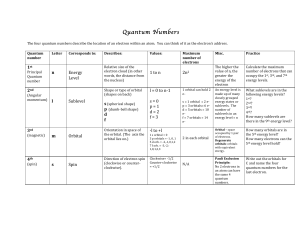
... The principal quantum number (n) cannot be zero. n must be 1, 2, 3, etc. The angular momentum quantum number (l ) can be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (ml) can be any integer between -l and +l. For l = 2, m can be eith ...
... The principal quantum number (n) cannot be zero. n must be 1, 2, 3, etc. The angular momentum quantum number (l ) can be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (ml) can be any integer between -l and +l. For l = 2, m can be eith ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
Atomic Orbitals and quantum numbers
... •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
... •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
Chapter 8 Notes - Bonding: General Concepts 8.1 Types of
... atoms are considered the best candidates 2. Only experimental evidence can conclusively determine the correct bonding situation in a molecule 8.13 Molecular Structure: The VSEPR Model A. Valence Shell Electron Pair Repulsion (VSEPR) 1. The structure around a given atom is determined principally by m ...
... atoms are considered the best candidates 2. Only experimental evidence can conclusively determine the correct bonding situation in a molecule 8.13 Molecular Structure: The VSEPR Model A. Valence Shell Electron Pair Repulsion (VSEPR) 1. The structure around a given atom is determined principally by m ...
Atomic Theory Study Guide - Reading Community Schools
... 6. Describe the photoelectric effect, including what changes in the effect result from variation of the incident light frequency and intensity and how it provides evidence for photons. 7. Calculate the energy of an emitted or absorbed photon from the initial and final energy levels of a molecule or ...
... 6. Describe the photoelectric effect, including what changes in the effect result from variation of the incident light frequency and intensity and how it provides evidence for photons. 7. Calculate the energy of an emitted or absorbed photon from the initial and final energy levels of a molecule or ...
photon may be totally absorbed by electron, but not have enough
... momentum of a particle. He proposed that only those orbits where the wave would be a circular standing wave will occur. This yields the same relation that Bohr had proposed. In addition, it makes more reasonable the fact that the electrons do not radiate, as one would otherwise expect from an accele ...
... momentum of a particle. He proposed that only those orbits where the wave would be a circular standing wave will occur. This yields the same relation that Bohr had proposed. In addition, it makes more reasonable the fact that the electrons do not radiate, as one would otherwise expect from an accele ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.





![ChemChapter_4[1]Light](http://s1.studyres.com/store/data/001894151_1-323884b777914f52c04d2bb917d4088a-300x300.png)