Chapter 4 - Rothschild Science
... What would happen if the frequency of the wave increased so much that you could hardly tell where one wave ended and another began? ...
... What would happen if the frequency of the wave increased so much that you could hardly tell where one wave ended and another began? ...
Document
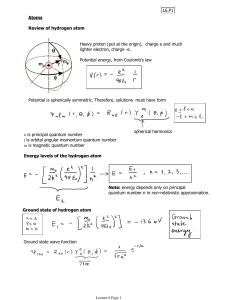
... 1. An electron in an atom moves in a circular orbit about the nucleus under the influence of the Coulomb attraction between the electron and the nucleus 2. The allowed orbit is a stationary orbit with a constant energy E 3. Electron radiates only when it makes a transition from one stationary state ...
... 1. An electron in an atom moves in a circular orbit about the nucleus under the influence of the Coulomb attraction between the electron and the nucleus 2. The allowed orbit is a stationary orbit with a constant energy E 3. Electron radiates only when it makes a transition from one stationary state ...
QUANTUM THEORY OF ATOMS AND MOLECULES
... energy from the ground to the first excited vibrational states. 3. Calculate the difference between the zero-point energy (in kJ mol) of C-H and C-D bond stretches, given a C-H vibrational stretching frequency of 2900 cm. Hence explain why C-H bonds react more rapidly than C-D bonds in many orga ...
... energy from the ground to the first excited vibrational states. 3. Calculate the difference between the zero-point energy (in kJ mol) of C-H and C-D bond stretches, given a C-H vibrational stretching frequency of 2900 cm. Hence explain why C-H bonds react more rapidly than C-D bonds in many orga ...
Unit 4 review sheet
... 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What four letters are used to represent the sublevels within a principle energy level? 40. What is the maximum number of electrons that may occupy one p orbital? 41. Who stated that ...
... 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What four letters are used to represent the sublevels within a principle energy level? 40. What is the maximum number of electrons that may occupy one p orbital? 41. Who stated that ...
The Atom and the Ion
... atom (give) are changed into a positive ion with equivalent number of positive charges to the given electrons. ...
... atom (give) are changed into a positive ion with equivalent number of positive charges to the given electrons. ...
Chapter 11 Notes
... In 1926, Erwin Schrodinger wrote a mathematical equation to describe the location and speed of an electron in an atom. This model is based on probability. This model describes the location in terms of the region in which one will find an electron 90% of the time. ...
... In 1926, Erwin Schrodinger wrote a mathematical equation to describe the location and speed of an electron in an atom. This model is based on probability. This model describes the location in terms of the region in which one will find an electron 90% of the time. ...
Models of the Atom
... Could not explain why emission lines are double, triple or more Could not explain why some lines brighter than others Could not explain how atoms bond Mixed classical and quantum ideas ...
... Could not explain why emission lines are double, triple or more Could not explain why some lines brighter than others Could not explain how atoms bond Mixed classical and quantum ideas ...
Many-Electron Atoms Thornton and Rex, Ch. 8
... In principle, can now solve Sch. Eqn for any atom. In practice, -> Complicated! ...
... In principle, can now solve Sch. Eqn for any atom. In practice, -> Complicated! ...
Electronic structure (download)
... We can predict the motion of a ball; But not an electron: problems locating small objects ...
... We can predict the motion of a ball; But not an electron: problems locating small objects ...
Chapt25_VGO
... Discrete spectra of atoms Existence of atoms Photo-electric effect (photons) Diffraction of electrons Classical physics was thought to describe the interaction of light with charges until there were discovered too many things that it could not explain. ...
... Discrete spectra of atoms Existence of atoms Photo-electric effect (photons) Diffraction of electrons Classical physics was thought to describe the interaction of light with charges until there were discovered too many things that it could not explain. ...
O Strong-Arming Electron Spin Dynamics
... ver ten years ago, Daniel Loss and David DiVincenzo proposed using the spin of a single electron as a quantum bit. At the time of the proposal, it was not possible to trap a single electron in a device and measure its spin, let alone demonstrate control of quantum coherence. In this talk I will desc ...
... ver ten years ago, Daniel Loss and David DiVincenzo proposed using the spin of a single electron as a quantum bit. At the time of the proposal, it was not possible to trap a single electron in a device and measure its spin, let alone demonstrate control of quantum coherence. In this talk I will desc ...
Shapes of the Charge Clouds
... The Hydrogen Atom and Quantum Theory Niels Bohr (Danish) tried to explain the spectrum of hydrogen atoms. •Energy is transferred in photon units (quanta), therefore specific amounts of energy are absorbed or emitted •Because the energy of an electron is quantized (discreet), there are only certain ...
... The Hydrogen Atom and Quantum Theory Niels Bohr (Danish) tried to explain the spectrum of hydrogen atoms. •Energy is transferred in photon units (quanta), therefore specific amounts of energy are absorbed or emitted •Because the energy of an electron is quantized (discreet), there are only certain ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.