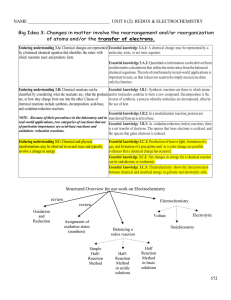
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Stoichiometry: Calculations with Chemical Formulas and
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Physics, Chemistry
... In this section, we examine how a set of base physical quantities and units is used to describe all other physical quantities. These precisely defined quantities and units, with accompanying order-of-ten prefixes (e.g. milli, centi and kilo) can then be used to describe the interactions between obje ...
... In this section, we examine how a set of base physical quantities and units is used to describe all other physical quantities. These precisely defined quantities and units, with accompanying order-of-ten prefixes (e.g. milli, centi and kilo) can then be used to describe the interactions between obje ...
File
... Some of the compounds in Table 20.11 are exceptions to the octet rule, like ICl 3. The row three halogens (Cl) and heavier (Br and I) have low lying empty d-orbitals available to expand their octet when they have to. Fluorine, with its valence electrons in the n = 2 level, does not have low energy d ...
... Some of the compounds in Table 20.11 are exceptions to the octet rule, like ICl 3. The row three halogens (Cl) and heavier (Br and I) have low lying empty d-orbitals available to expand their octet when they have to. Fluorine, with its valence electrons in the n = 2 level, does not have low energy d ...
Physics, Biology
... In this section, we examine how a set of base physical quantities and units is used to describe all other physical quantities. These precisely defined quantities and units, with accompanying order-of-ten prefixes (e.g. milli, centi and kilo) can then be used to describe the interactions between obje ...
... In this section, we examine how a set of base physical quantities and units is used to describe all other physical quantities. These precisely defined quantities and units, with accompanying order-of-ten prefixes (e.g. milli, centi and kilo) can then be used to describe the interactions between obje ...
Learning Outcomes
... (b) describe, with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shells (energy levels) (Knowledge of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ...
... (b) describe, with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shells (energy levels) (Knowledge of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ...
Topic 1: Quantitative chemistry (12
... transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of ...
... transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of ...
Topic 1: Quantitative chemistry (12
... transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of ...
... transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of ...
Chemistry I Honors Semester I FINAL EXAM REVIEW Atomic
... Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Which of the following is an extensive property of matter? a. melting point b. boiling point c. volume d. density ____ 2. An atom is a. the smallest unit of matter that maintains its chemical ident ...
... Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Which of the following is an extensive property of matter? a. melting point b. boiling point c. volume d. density ____ 2. An atom is a. the smallest unit of matter that maintains its chemical ident ...
Chapter 2 Matter and Components F11 110
... is found in a fixed amount in nature, and rarely are these amounts equal among the given isotopes of an element we must have a way to take this into account when talking about a naturally occurring element; enter Average Mass: ...
... is found in a fixed amount in nature, and rarely are these amounts equal among the given isotopes of an element we must have a way to take this into account when talking about a naturally occurring element; enter Average Mass: ...
Chapter 2 Matter and Components F11 110pt
... 1. Some compounds have been known and used for so long that their trivial (or common names) have become accepted by the IUPAC as official: ...
... 1. Some compounds have been known and used for so long that their trivial (or common names) have become accepted by the IUPAC as official: ...
Slide 1
... • Thomson found that the mass to charge ratio of the electron was -5.686x10-12 kg/C • Millikan found the charge on the electron was -1.62 x 10-19 C. (C is the abbreviation for the coulomb, a unit of charge.) • Therefore the mass of the electron can be calculated: mass = charge x mass/charge • mass ...
... • Thomson found that the mass to charge ratio of the electron was -5.686x10-12 kg/C • Millikan found the charge on the electron was -1.62 x 10-19 C. (C is the abbreviation for the coulomb, a unit of charge.) • Therefore the mass of the electron can be calculated: mass = charge x mass/charge • mass ...
cont. - Appoquinimink High School
... • What we call the atomic weight on the periodic table is actually the average atomic mass of that element’s naturally occurring isotopes. • Isotopes have similar chemical properties in that they combine with other elements to form similar compounds. (cont.) © 2004 Key Curriculum Press. ...
... • What we call the atomic weight on the periodic table is actually the average atomic mass of that element’s naturally occurring isotopes. • Isotopes have similar chemical properties in that they combine with other elements to form similar compounds. (cont.) © 2004 Key Curriculum Press. ...
chemistry (9189)
... industrial and laboratory visits relevant to the content of the options chosen. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, learning Outcomes have been used throughout. Each part of the syllabus is specified by a Content ...
... industrial and laboratory visits relevant to the content of the options chosen. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, learning Outcomes have been used throughout. Each part of the syllabus is specified by a Content ...
Atomic Theory of Matter
... Rules for predicting charges on monatomic ions Most of the main group metals form cations with the charge equal to their group number. The charge on a monatomic anion for a nonmetal equals the group number minus 8. Most transition elements form more than one ion, each with a different charge. (See T ...
... Rules for predicting charges on monatomic ions Most of the main group metals form cations with the charge equal to their group number. The charge on a monatomic anion for a nonmetal equals the group number minus 8. Most transition elements form more than one ion, each with a different charge. (See T ...
Atomic Models 100
... Answer =In an atom, the central core that contains most of the atom’s mass. Protons and neutrons are located there. Back to Main ...
... Answer =In an atom, the central core that contains most of the atom’s mass. Protons and neutrons are located there. Back to Main ...