Thermochemistry
... Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. ...
... Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. Calorimetry is the science of measuring the heat of chemical reactions or physical changes. ...
Chemistry Unit 5 Test Review The Mole and Balancing Equations
... 6. Two alcohols that are used in our everyday lives are rubbing alcohol and ethylene glycol. Rubbing alcohol is used as an antiseptic. Ethylene glycol is the main ingredient in antifreeze, which is used in automobile cooling systems. What is the molecular weight of ethylene glycol, C 2 H4 (OH) 2 ? W ...
... 6. Two alcohols that are used in our everyday lives are rubbing alcohol and ethylene glycol. Rubbing alcohol is used as an antiseptic. Ethylene glycol is the main ingredient in antifreeze, which is used in automobile cooling systems. What is the molecular weight of ethylene glycol, C 2 H4 (OH) 2 ? W ...
Lecture 6 – Thermochemistry
... species must be stated 4. The value of ΔH applies when products and reactants are at the same temperature, usually 25 °C ...
... species must be stated 4. The value of ΔH applies when products and reactants are at the same temperature, usually 25 °C ...
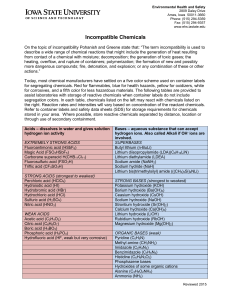
Incompatible Chemicals
... 2809 Daley Drive Ames, Iowa 50011-3660 Phone: (515) 294-5359 Fax: (515) 294-9357 www.ehs.iastate.edu ...
... 2809 Daley Drive Ames, Iowa 50011-3660 Phone: (515) 294-5359 Fax: (515) 294-9357 www.ehs.iastate.edu ...
Document
... Some reactions require more energy than is produced; others produce more energy than is ...
... Some reactions require more energy than is produced; others produce more energy than is ...
Chapters 9 and 10
... Indicate the total number of sigma (σ) bonds and the total number of pi (π) bonds in the molecule ...
... Indicate the total number of sigma (σ) bonds and the total number of pi (π) bonds in the molecule ...
Two moles of gas at 1 bar and 298 K are compressed at constant T
... • There are situations where we would like to know the heat of a reaction at other than 298 with high accuracy. • For example, in industrial large scale reactions heat from an exothermic reaction must be accurately known to design the correct cooling devices so that reactors do not overheat and cau ...
... • There are situations where we would like to know the heat of a reaction at other than 298 with high accuracy. • For example, in industrial large scale reactions heat from an exothermic reaction must be accurately known to design the correct cooling devices so that reactors do not overheat and cau ...
Lecture notes Chapters 10
... are maximized. In unsaturated fatty acids, the cis double bonds interrupt the regular packing of the chains and the London dispersion forces is smaller. COOH COOH ...
... are maximized. In unsaturated fatty acids, the cis double bonds interrupt the regular packing of the chains and the London dispersion forces is smaller. COOH COOH ...
Chemicals: What`s in? What`s out?
... Mention the word chemistry in a middle level classroom and the first thing students want to know is, “Will we be blowing anything up?” Chemistry should be fun and exciting, but much preparation and skill are needed by the teacher and students in working with chemicals. Unfortunately, accidents do ha ...
... Mention the word chemistry in a middle level classroom and the first thing students want to know is, “Will we be blowing anything up?” Chemistry should be fun and exciting, but much preparation and skill are needed by the teacher and students in working with chemicals. Unfortunately, accidents do ha ...
2007 - SolPass
... Which of these is the percent of error in evaluating the molecular mass of a compound if the experimental value was 105.2 amu and the known value was ...
... Which of these is the percent of error in evaluating the molecular mass of a compound if the experimental value was 105.2 amu and the known value was ...
Direct production of hydrogen peroxide from CO, O2, and H2O over
... Table 1 shows the catalytic results for H2O2 production from CO/O2/H2O over several types of metal nanoparticles dispersed on alumina prepared by the wet reduction (WR) method, which has recently been shown to be an effective method for the preparation of various amorphous alloy catalysts for versati ...
... Table 1 shows the catalytic results for H2O2 production from CO/O2/H2O over several types of metal nanoparticles dispersed on alumina prepared by the wet reduction (WR) method, which has recently been shown to be an effective method for the preparation of various amorphous alloy catalysts for versati ...
Chemistry EOC Review Name
... 104. Identify the name of the following phase changes (If you forget on EOC, consider water and its changes) 105. a. solid to liquid b. gas to liquid c. liquid to solid d. solid to gas 106. How are the pressure and volume of a gas related? 107. A gas is originally at a volume of 6 mL and a pressure ...
... 104. Identify the name of the following phase changes (If you forget on EOC, consider water and its changes) 105. a. solid to liquid b. gas to liquid c. liquid to solid d. solid to gas 106. How are the pressure and volume of a gas related? 107. A gas is originally at a volume of 6 mL and a pressure ...
Redox Reactions - KFUPM Faculty List
... Oxidation-reduction reactions (sometimes called redox reactions)) are reactions involvingg the transfer of one electron or more from one reactant to another. Redox reaction also involves the change in oxidation states for molecules. These reactions are very common in life: • Photosynthesis. (convers ...
... Oxidation-reduction reactions (sometimes called redox reactions)) are reactions involvingg the transfer of one electron or more from one reactant to another. Redox reaction also involves the change in oxidation states for molecules. These reactions are very common in life: • Photosynthesis. (convers ...
Semester 2 Review WS
... 4. Name ionic and molecular compounds from formulas and vice versa. Write the formula of the following compounds. Circle the ionic compounds! Underline polyatomic ions. a potassium iodide ...
... 4. Name ionic and molecular compounds from formulas and vice versa. Write the formula of the following compounds. Circle the ionic compounds! Underline polyatomic ions. a potassium iodide ...
(p. 522)
... 5. Which of the following statements about the effective nuclear charge, Zeff, is correct? (p. 554) C A.Zeff increases with the size of the atom. B.Zeff decreases across a period and increases down a group. C.Zeff increases across a period and is relatively constant down a group. D.Zeff increases as ...
... 5. Which of the following statements about the effective nuclear charge, Zeff, is correct? (p. 554) C A.Zeff increases with the size of the atom. B.Zeff decreases across a period and increases down a group. C.Zeff increases across a period and is relatively constant down a group. D.Zeff increases as ...
Document
... impurities are carbon, silicon and phosphorus. The diagram below shows one method of making steel from iron. oxygen and powdered ...
... impurities are carbon, silicon and phosphorus. The diagram below shows one method of making steel from iron. oxygen and powdered ...
ALE 23. Balancing Redox Reactions
... dichromate and the endpoint has been reached. What is the molarity of the original Na2SO3(aq) solution? Show your work. ...
... dichromate and the endpoint has been reached. What is the molarity of the original Na2SO3(aq) solution? Show your work. ...
Lab 1-1 - My eCoach
... INTRODUCTION: Chemistry is a science that investigates changes in matter. Chemical reactions are the changes matter undergoes. The changes you can observe are called “macroscopic changes.” Often these changes, such as color changes, the formation of a solid (precipitation), or the formation of gas b ...
... INTRODUCTION: Chemistry is a science that investigates changes in matter. Chemical reactions are the changes matter undergoes. The changes you can observe are called “macroscopic changes.” Often these changes, such as color changes, the formation of a solid (precipitation), or the formation of gas b ...
CLASS X carbon and its compound
... carbon atoms by a single covalent bond are called branched chain hydrocarbons. 13. Isomerism : The phenomenon due to which there can exist two or more organic compounds, with different physical and chemical properties, due to the difference in arrangement of carbon atoms in their structure, but have ...
... carbon atoms by a single covalent bond are called branched chain hydrocarbons. 13. Isomerism : The phenomenon due to which there can exist two or more organic compounds, with different physical and chemical properties, due to the difference in arrangement of carbon atoms in their structure, but have ...
1. Write the balanced equation for the combustion of butane (C4H10
... 3. Potassium chloride is formed from its constituent elements. How many grams of KCl is produced from 2.50 g of K and excess Cl2. From 1.00 g of Cl2 and excess K? 4. Sodium oxide and water react in a synthesis reaction. How many grams of NaOH is produced from 1.20 x 102 grams of Na2O? How many grams ...
... 3. Potassium chloride is formed from its constituent elements. How many grams of KCl is produced from 2.50 g of K and excess Cl2. From 1.00 g of Cl2 and excess K? 4. Sodium oxide and water react in a synthesis reaction. How many grams of NaOH is produced from 1.20 x 102 grams of Na2O? How many grams ...
GC97F Pretest A - American Chemical Society
... (C) A catalyst provides additional energy to a reactant so it can achieve the necessary activation energy. (D) A catalyst provides an alternative reaction pathway with a lower activation energy. Questions 31 and 32 should be answered with reference to the equation 2 C(s) + O2(g) 2 CO(g) ∆H < 0 31. W ...
... (C) A catalyst provides additional energy to a reactant so it can achieve the necessary activation energy. (D) A catalyst provides an alternative reaction pathway with a lower activation energy. Questions 31 and 32 should be answered with reference to the equation 2 C(s) + O2(g) 2 CO(g) ∆H < 0 31. W ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.