* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 6 – Thermochemistry
Photoredox catalysis wikipedia , lookup
Rate equation wikipedia , lookup
Marcus theory wikipedia , lookup
Electrolysis of water wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Solar air conditioning wikipedia , lookup
Click chemistry wikipedia , lookup
Thermodynamics wikipedia , lookup
George S. Hammond wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Heat transfer wikipedia , lookup
Transition state theory wikipedia , lookup
THERMOCHEMISTRY
Study of heat change in chemical
reactions
Systems, Surroundings & Internal
Energy
System = the part of the universe under study
chemicals in a flask.
the coffee in your coffee cup.
my textbook.
Surroundings = rest of the universe (or as much as
needed…)
the flask.
perhaps the flask and this classroom.
perhaps the flask and all of the building, etc.
Universe = System + Surroundings
The First Law of Thermodynamics
• Thermodynamics
• Deals with all kinds of energy effects in all
kinds of processes
Two types of energy:
• Heat (q)
• Work (w)
The Law of Conservation of Energy
• ΔEsystem = - ΔEsurroundings
The First Law:
• ΔE = q + w
• The total change in energy is equal to the sum
of the heat and work transferred between the
system and the surroundings
The First Law of
Thermodynamics
Internal energy = E within the system because of
nanoscale position or motion
• Conservation of energy becomes:
ΔE = q + w
Heat
• Thermal E transfer caused by a T difference.
• Heat flows from hotter to cooler objects until
they reach thermal equilibrium (have equal T
).
Work
• In an internal combustion engine, a
significant fraction of the energy of
combustion is converted to useful work
– The expansion of the combustion gases
produces a volume and a pressure change
– The system does work on its surroundings
• Propels the car forward
• Overcomes friction
• Charges battery
PressureVolume Work
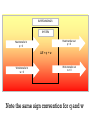
SURROUNDINGS
SYSTEM
Heat transfer out
q<0
Heat transfer in
q>0
ΔE = q + w
Work transfer in
w>0
Work transfer out
w<0
Note the same sign convention for q and w
SAMPLE PROBLEMS
1. A liquid cools from 45°C to 30°C,
transferring 911 J to the surroundings. No work
is done on or by the liquid. What is ΔEliquid?
2. A system does 50.2 J of work on its surroundings
and there is a simultaneous 90.1 J heat transfer from
the surroundings to the system. What is ΔEsystem?
State Functions and Path
Independence
State functions
Always have the same value whenever
the system is in the same state.
State
functions
H
P
Two equal mass samples of water produced by:
T
1. Heating one from 20°C to 50°C.
2. Cooling the other from 100°C to 50°C.
have identical final H (and V, P, E…).
State function changes are path
independent.
ΔH = Hfinal – Hinitial is constant.
E
V
etc.
ENTHALPY of Chemical
Reactions
• At constant V,
ΔV = 0
• At constant p,
Enthalpy, ΔH
• H = E + PV
• ΔH = ΔE + PΔV ; ΔH = qp
– The PV product is important only where
gases are involved; it is negligible when
only liquids or solids are involved
• ΔH = ΔE + ΔngRT
– Δng is the change in the number of moles of
gas as the reaction proceeds
Measurement of Heat Flow:
Calorimetry
• A calorimeter is a device used to
measure the heat flow of a reaction
– The walls of the calorimeter are insulated to
block heat flow between the reaction and the
surroundings
– The heat flow for the system is equal in
magnitude and opposite in sign from the
heat flow of the calorimeter
• qreaction = - qcalorimeter
• qreaction = - Ccal Δt
The Calorimetry Equation
q = C x Δt
– Δt = tfinal – tinitial
– C (uppercase) is the heat capacity of the
system: it is the quantity of heat needed to raise
the temperature of the system by 1 °C
q = m x c x Δt
– c (lowercase) is the specific heat: the quantity
of heat needed to raise the temperature of one
gram of a substance by 1 °C
• c depends on the identity and phase of the
substance
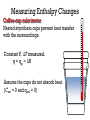
Measuring Enthalpy Changes
Coffee-cup calorimeter
Nested styrofoam cups prevent heat transfer
with the surroundings.
Constant P. ΔT measured.
q = qp = ΔH
Assume the cups do not absorb heat.
(Ccal = 0 and qcal = 0)
Measuring Enthalpy Changes
Bomb Calorimeter
Measure ΔT of the water. Constant V:
Conservation of E:
qreaction + qbomb + qwater = 0
or
−qreaction = qbomb + qwater
with
qbomb = mcalccalΔT = CcalΔT
A constant for a calorimeter
qV = ΔE
Sample Problem
Copper is used in building the
integrated circuits, chips and printed
circuit boards for computers. When 228 J
of heat are absorbed by 125 g of copper
at 22.38oC, the temperature increases to
27.12oC. What is the specific heat of
copper?
A student wishes to determine the heat
capacity of a coffee-cup calorimeter.
After she mixes 100.0 g of water at
58.5°C with 100.0 g of water, already in
the calorimeter, at 22.8°C, the final
temperature of the water is 39.7°C.
Calculate the heat capacity of the
calorimeter in J/°C. Use 4.184 J/g - °C
as the specific heat of water.
Measuring Enthalpy Changes
Octane (0.600 g) was burned in a bomb calorimeter
containing 751 g of water. T increased from 22.15°C to
29.12°C. Calculate the heat evolved per mole of octane
burned. Ccal = 895 J°C-1.
2 C8H18(l) + 25 O2(g)
16 CO2(g) + 18 H2O(l)
qsys + qsurr = 0
qreaction + qbomb + qwater = 0
qbomb = CcalΔT = 895 J°C-1 (29.12 – 22.15)°C = +6238 J
qwater = m c ΔT = 751 g (4.184 J g-1 °C-1)(29.12 – 22.15)°C
= +2.190 x 104 J
So −qreaction = +6238 + 2.190 x 104 J = 2.81 x 104 J = 28.1 kJ
qreaction = −28.1 kJ
Measuring Enthalpy Changes
Octane (0.600 g) was burned in a bomb calorimeter… Calculate the heat
evolved per mole of octane burned
Molar mass of C8H18 = 114.23 g/mol.
nC8H18 = (0.600 g) / (114.23 g/mol)
= 0.00525 mol C8H18
Heat evolved /mol octane = −28.1 kJ
0.00525 mol
= −5.35 x 103 kJ/mol
= −5.35 MJ/mol
Thermochemical Equations
• a chemical equation with the ΔH for
the reaction included
Example
NH4NO3 (s) NH4+ (aq) + NO3- (aq) ΔH = +28.1kJ
This means:
+28.1 kJ
+28.1 kJ
Or
1 mol NH4NO3(s) 1 mol NH4+(aq)
+28.1 kJ
or
1 mol NO3-(aq)
Conventions for
Thermochemical Equations
1. The sign of ΔH indicates whether the
reaction is endothermic or exothermic
2. The coefficients of the thermochemical
equation represent the number of moles of
reactant and product
3. The phases of all reactant and product
species must be stated
4. The value of ΔH applies when products and reactants
are at the same temperature, usually 25 °C
Rules of Thermochemistry
• ΔH is directly proportional to the amount
of reactant or product
– If a reaction is divided by 2, so is ΔH
– If a reaction is multiplied by 6, so is ΔH
• ΔH changes sign when the reaction is
reversed
• ΔH has the same value regardless of the
number of steps
Sample Problem
In photosynthesis, the following reaction takes place:
6CO2(g) + 6H2O(l) 6O2(g) + C6H12O6(s) H = + 2801 KJ
a. Calculate H when one mole of carbon dioxide
reacts?
b. How many kilojoules of energy are liberated when
15.00 g of glucose is burned in oxygen?
METHODS OF DETERMINING
Hreaction
1. DIRECT METHOD
- using Hof of every reactants and
products of chemical reaction
2. INDIRECT METHOD
- using HESS LAW (for reaction
involving several steps)
DIRECT METHOD
ΔH° ={(nproducts)(ΔHf° products)}
– {(nreactants)(ΔHf° reactants)}
Where: ΔHf° = standard molar enthalpy of
formation taken from Thermodynamic data
Standard Molar Enthalpy of
Formation
Standard state = most stable form of the pure
element at P = 1 bar.
e.g. C standard state = graphite (not diamond)
ΔHf° for any element in its standard state is zero.
(take 1 mol of the element and make… 1 mol of element)
ΔHf° (Br2(l) ) = 0
ΔHf° (Br2(g) ) ≠ 0
at 298 K
at 298 K
Formation reaction
Make 1 mol of compound from its elements in their
standard states.
H2 combustion:
2 H2(g) + O2(g)
2 H2O(l)
ΔH° = −571.66 kJ
but the formation reaction is:
H2(g) + ½ O2(g)
1H2O(l)
ΔHf° = −285.83 kJ
f = formation
DIRECT METHOD
ΔH° ={(nproducts)(ΔHf° products)}
– {(nreactants)(ΔHf°
reactants)}
Example
Calculate ΔH° for:
CH4(g) + NH3(g) HCN(g) + 3 H2(g)
ΔHf° : -46.11 -74.85
+134
0
ΔH° = [1ΔHf°(HCN) + 3 ΔHf°(H2)] −
[1ΔHf°(NH3) +
1ΔHf°(CH4)]
= [+134 + 3(0)] − [− 46.11 + (−74.85)] = 255 kJ
INDIRECT METHOD Hess’s Law
“If the equation for a reaction is the sum of the
equations for two or more other reactions, then
ΔH° for the 1st reaction must be the sum of the
ΔH° values of the other reactions.”
Another version:
“ΔH° for a reaction is the same whether it takes
place in a single step or several steps.”
H is a state function
SAMPLE PROBLEMS




























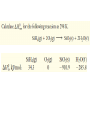


![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-150x150.png)










