Student Solutions Manual Errata
... determine the states for the products. If one or both products is insoluble, is a gas, or is a molecule (for example, water), a reaction takes place. (a) Reactants: Sr(NO3)2(aq) and H2SO4(aq) Ions: Sr2+, NO3–, H+, SO42– Products: SrSO4 and HNO3 Solubility (Table 5.3, Table 3.10): Sr(NO3)2(aq), H2SO4 ...
... determine the states for the products. If one or both products is insoluble, is a gas, or is a molecule (for example, water), a reaction takes place. (a) Reactants: Sr(NO3)2(aq) and H2SO4(aq) Ions: Sr2+, NO3–, H+, SO42– Products: SrSO4 and HNO3 Solubility (Table 5.3, Table 3.10): Sr(NO3)2(aq), H2SO4 ...
Chemistry Tests Questions
... 14. What important property increases as the vertical arrangement of the noble (inert) gases is descended? 15. What is the family name of the group I elements? 16. Are the group one elements soft or hard? 17. How can you prevent the corrosion of sodium? 18. Which of the group I elements reacts most ...
... 14. What important property increases as the vertical arrangement of the noble (inert) gases is descended? 15. What is the family name of the group I elements? 16. Are the group one elements soft or hard? 17. How can you prevent the corrosion of sodium? 18. Which of the group I elements reacts most ...
9.5. Combined Methods: Electrochemical
... 9.2. Chemical Modification Methods Some of the chemical modification methods have been investigated for many years, even though the analytical aspects were not envisaged initially. The simplest technique involves the treatment with an oxidizing acid solution such as nitric acid or chromic acid. ...
... 9.2. Chemical Modification Methods Some of the chemical modification methods have been investigated for many years, even though the analytical aspects were not envisaged initially. The simplest technique involves the treatment with an oxidizing acid solution such as nitric acid or chromic acid. ...
111 Exam IV outline
... 1. The forward reaction (⇀ ) and reverse (↽ ) reactions are occurring simultaneously. 2. The rate for the forward reaction is equal to the rate of the reverse reaction and a dynamic equilibrium is achieved. 3. The ratio of the concentrations of the products to reactants is constant. B. THE EQUILIBRI ...
... 1. The forward reaction (⇀ ) and reverse (↽ ) reactions are occurring simultaneously. 2. The rate for the forward reaction is equal to the rate of the reverse reaction and a dynamic equilibrium is achieved. 3. The ratio of the concentrations of the products to reactants is constant. B. THE EQUILIBRI ...
Part V The Third Law and Free Energy
... We have seen that the second law of thermodynamics does not enable the absolute value of entropy of any substance to be calculated; this law only permits evaluation of entropy changes for specified changes in physical or chemical state. More precisely, the second law provides a complete description ...
... We have seen that the second law of thermodynamics does not enable the absolute value of entropy of any substance to be calculated; this law only permits evaluation of entropy changes for specified changes in physical or chemical state. More precisely, the second law provides a complete description ...
CHE 110 Dr. Nicholas Bizier Office DS 337b email
... Lysine is an amino acid which has the following elemental composition: C, H, O, N. In one experiment, 2.175 g of lysine was combusted to produce 3.94 g of CO2 and 1.89 g H2O. In a separate experiment, 1.873 g of lysine was burned to produce 0.436 g of NH2. The molar mass of lysine is 150 g/mol. Dete ...
... Lysine is an amino acid which has the following elemental composition: C, H, O, N. In one experiment, 2.175 g of lysine was combusted to produce 3.94 g of CO2 and 1.89 g H2O. In a separate experiment, 1.873 g of lysine was burned to produce 0.436 g of NH2. The molar mass of lysine is 150 g/mol. Dete ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
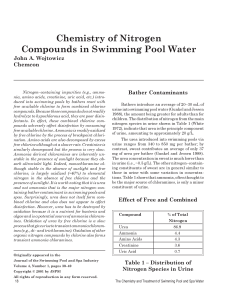
... A buffer solution is one that maintains a fairly constant pH despite the addition of small amounts of either acid or base. When acid is added, the hydrogen ions added are removed as they react with the methanoate ions: HCOO−(aq) + H+(aq) → HCOOH(aq) When base is added, the hydroxide ions added are r ...
... A buffer solution is one that maintains a fairly constant pH despite the addition of small amounts of either acid or base. When acid is added, the hydrogen ions added are removed as they react with the methanoate ions: HCOO−(aq) + H+(aq) → HCOOH(aq) When base is added, the hydroxide ions added are r ...
PDF (chapter_8)
... Johnson et al.14. The drift length of the ion mobility spectrometer was 13.65 cm and was operated in the positive mode. A drift voltage of 3988 V, corresponding to electric field strength of 292 V/cm, was employed. All measurements were made at local atmospheric pressure (~730 Torr) while a counter ...
... Johnson et al.14. The drift length of the ion mobility spectrometer was 13.65 cm and was operated in the positive mode. A drift voltage of 3988 V, corresponding to electric field strength of 292 V/cm, was employed. All measurements were made at local atmospheric pressure (~730 Torr) while a counter ...
Chapter 3 Electric Potential
... Notice that for the path A → C → B , work is done by the field only along the segment AC which is parallel to the field lines. Points B and C are at the same electric potential, i.e., VB = VC . Since ∆U = q∆V , this means that no work is required in moving a charge from B to C. In fact, all points a ...
... Notice that for the path A → C → B , work is done by the field only along the segment AC which is parallel to the field lines. Points B and C are at the same electric potential, i.e., VB = VC . Since ∆U = q∆V , this means that no work is required in moving a charge from B to C. In fact, all points a ...
Gas-Phase Reactions of Fe (CH2O)+ and Fe (CH2S)+ with Small
... been the focus of intense investigation for the past 20 years, yielding a great deal of information on “intrinsic” properties, such as kinetics, thermochemistry, and reaction mechanisms in the absence of solvation and counterion effects.1 The reactions with simple hydrocarbons have been particularly ...
... been the focus of intense investigation for the past 20 years, yielding a great deal of information on “intrinsic” properties, such as kinetics, thermochemistry, and reaction mechanisms in the absence of solvation and counterion effects.1 The reactions with simple hydrocarbons have been particularly ...
unit 7 hw packet File - District 196 e
... Sodium metal reacts with water to produce sodium hydroxide and hydrogen gas according to the following equation: 2Na(s) + 2H2O(l) 2(g) a. How many moles of sodium are consumed if 92 grams of sodium metal react with excess water? ...
... Sodium metal reacts with water to produce sodium hydroxide and hydrogen gas according to the following equation: 2Na(s) + 2H2O(l) 2(g) a. How many moles of sodium are consumed if 92 grams of sodium metal react with excess water? ...
Kinetic study on carbonation of crude Li2CO3 with CO2
... High purity Li2CO3 can be used for the production of medicines, electronic grade crystals, such as LiNbO3 and LiTaO3 crystals, and high purity lithium metal. With the development of batteries and single crystal industries in the world, the demand for it has been dramatically growing in recent years, ...
... High purity Li2CO3 can be used for the production of medicines, electronic grade crystals, such as LiNbO3 and LiTaO3 crystals, and high purity lithium metal. With the development of batteries and single crystal industries in the world, the demand for it has been dramatically growing in recent years, ...
Answers - logo Pre-U Chemistry Textbook
... The two things that affect the size of hydration energies are ionic radius and the charge on the ion. The higher the charge on the ion the more exothermic ∆hydrH. The value for Mg2+ is nearly five times as large as Na+. Al3+ is nearly two and a half times as big as Mg2+. By comparing the values for ...
... The two things that affect the size of hydration energies are ionic radius and the charge on the ion. The higher the charge on the ion the more exothermic ∆hydrH. The value for Mg2+ is nearly five times as large as Na+. Al3+ is nearly two and a half times as big as Mg2+. By comparing the values for ...
STUDY MATERIAL 2015-16 CHEMISTRY CLASS XI
... definite proportion by mass, which could be split by suitable chemical methods. Characteristics of compound Compounds always contain a definite proportion of the same elements by mass. The properties of compounds are totally different from the elements from which they are formed. Compounds are homog ...
... definite proportion by mass, which could be split by suitable chemical methods. Characteristics of compound Compounds always contain a definite proportion of the same elements by mass. The properties of compounds are totally different from the elements from which they are formed. Compounds are homog ...
Syllabus - Chemistry
... teachers of the departments followed by an open viva-voce session. This would be conducted by a team of three teachers, one from each branch, who would award marks out of 25 based on the presentation and performance in question answer session. ...
... teachers of the departments followed by an open viva-voce session. This would be conducted by a team of three teachers, one from each branch, who would award marks out of 25 based on the presentation and performance in question answer session. ...
Multipole Expansion of the Electrostatic Potential
... 2.1 Find the dipole moment of the system of four point charges q at (a, 0, 0), q at (0, a, 0), −q at (−a, 0, 0) and −q at (0, −a, 0). 2.2 Write the potential for the system of three point charges: two charges +q in the points (0, 0, a) and (0, 0, −a), and a charge −2q in the origin of the frame. Fin ...
... 2.1 Find the dipole moment of the system of four point charges q at (a, 0, 0), q at (0, a, 0), −q at (−a, 0, 0) and −q at (0, −a, 0). 2.2 Write the potential for the system of three point charges: two charges +q in the points (0, 0, a) and (0, 0, −a), and a charge −2q in the origin of the frame. Fin ...
纳米结构体系物理化学性质的理论研究方法与实例
... and those moving in the opposite direction. We designate the concentration of these by Nf‡ and Nb‡, respectively. At equilibrium the rate of the forward reaction must be the same as the backward reaction; accordingly, the concentrations of the TSs moving toward reactants and toward products must be ...
... and those moving in the opposite direction. We designate the concentration of these by Nf‡ and Nb‡, respectively. At equilibrium the rate of the forward reaction must be the same as the backward reaction; accordingly, the concentrations of the TSs moving toward reactants and toward products must be ...
CHAPTER 16
... The quantity of energy transferred as heat during a temperature change depends on the nature of the material changing temperature, the mass of the material changing temperature, and the size of the temperature change. One gram of iron heated to 100.0°C and cooled to 50.0°C in a calorimeter transfers ...
... The quantity of energy transferred as heat during a temperature change depends on the nature of the material changing temperature, the mass of the material changing temperature, and the size of the temperature change. One gram of iron heated to 100.0°C and cooled to 50.0°C in a calorimeter transfers ...
2 - C7Chemistry
... tetrachloromethane and hydrogen chloride gas will be produced. How many L of methane will react with 0.800 L of chlorine gas at STP? 1 L CH 4 0.800 L Cl2 0.200 L Cl2 4 L Cl2 ...
... tetrachloromethane and hydrogen chloride gas will be produced. How many L of methane will react with 0.800 L of chlorine gas at STP? 1 L CH 4 0.800 L Cl2 0.200 L Cl2 4 L Cl2 ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.