Thermodynamics and Equilibrium
... spontaneously because doing so lowers its energy, but it does not move back up the hill spontaneously because an input of energy is required to do so. Thus, it would be tempting to conclude that processes should be spontaneous if they are exothermic. However, there are many examples of spontaneous e ...
... spontaneously because doing so lowers its energy, but it does not move back up the hill spontaneously because an input of energy is required to do so. Thus, it would be tempting to conclude that processes should be spontaneous if they are exothermic. However, there are many examples of spontaneous e ...
Topic 5 Energetics File
... Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stab ...
... Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stab ...
Chemistry
... Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The microscopic level looks at the structure of matter that gives rise to these interactions. At O-Level, students have been intr ...
... Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The microscopic level looks at the structure of matter that gives rise to these interactions. At O-Level, students have been intr ...
Chemical Reactions and Stoichiometry
... are many other chemical reactions that occur during the cooking of a steak as well. When you heat food that contains both protein and carbohydrates (sugars), a complex set of reactions occur—the Maillard r eaction—to form many flavourful and aromatic compounds. In fact, the Maillard reaction is ext ...
... are many other chemical reactions that occur during the cooking of a steak as well. When you heat food that contains both protein and carbohydrates (sugars), a complex set of reactions occur—the Maillard r eaction—to form many flavourful and aromatic compounds. In fact, the Maillard reaction is ext ...
PREPARATIONS AND APPLICATION OF METAL NANOPARTICLES
... Polymer in solid state or resin supports might have different properties in comparison to the conventional support such as alumina, titania, silica, etc. The particles are inside polymer matrix, and not simply on the surface of the support particles as is common case in the inorganic support. The pa ...
... Polymer in solid state or resin supports might have different properties in comparison to the conventional support such as alumina, titania, silica, etc. The particles are inside polymer matrix, and not simply on the surface of the support particles as is common case in the inorganic support. The pa ...
PURPOSE: To determine the value of the equilibrium constant for a
... 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How would the slope of the calibration curve (absorbance on the y-axis versus concentration of Fe(SCN)2+on the x-axis) be affected? How would this impact your Kc in Part C ...
... 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How would the slope of the calibration curve (absorbance on the y-axis versus concentration of Fe(SCN)2+on the x-axis) be affected? How would this impact your Kc in Part C ...
Chemistry - Sanskriti School
... (elementary idea). Buffer solutions, solubility product, common . ion effect (with illustrative examples). Unit V: States of Matter: Gases and Liquids. Three states of matter. Intermolecular interactions, type of bonding, melting and boiling points. Role of gas laws in elucidating the concept of the ...
... (elementary idea). Buffer solutions, solubility product, common . ion effect (with illustrative examples). Unit V: States of Matter: Gases and Liquids. Three states of matter. Intermolecular interactions, type of bonding, melting and boiling points. Role of gas laws in elucidating the concept of the ...
Electric Potential Maps and Voltage
... In our discussion of Bernoulli’s equation, we gave the collection of terms (P + ρgh + 1/2ρv2 ) the name hydrodynamic voltage. The content of Bernoulli’s equation is that this hydrodynamic voltage is constant along a stream line when the fluid is incompressible and viscous forces can be neglected. Tw ...
... In our discussion of Bernoulli’s equation, we gave the collection of terms (P + ρgh + 1/2ρv2 ) the name hydrodynamic voltage. The content of Bernoulli’s equation is that this hydrodynamic voltage is constant along a stream line when the fluid is incompressible and viscous forces can be neglected. Tw ...
LECTURE PPT: Chapter 8
... • In the case of octane, a shortage of O2 causes side reactions that result in pollutants such as carbon monoxide (CO) and ozone. • The 1990 amendments to the Clean Air Act required oil companies to put additives in gasoline that increased its oxygen content. © 2015 Pearson Education, Inc. ...
... • In the case of octane, a shortage of O2 causes side reactions that result in pollutants such as carbon monoxide (CO) and ozone. • The 1990 amendments to the Clean Air Act required oil companies to put additives in gasoline that increased its oxygen content. © 2015 Pearson Education, Inc. ...
Chemical Quantities and Aqueous Reactions
... The amount of carbon dioxide emitted by fossil fuel combustion is related to the amount of fossil fuel that is burned—the balanced chemical equations for the combustion reactions give the exact relationships between these amounts. In this discussion, we use octane (a component of gasoline) as a repr ...
... The amount of carbon dioxide emitted by fossil fuel combustion is related to the amount of fossil fuel that is burned—the balanced chemical equations for the combustion reactions give the exact relationships between these amounts. In this discussion, we use octane (a component of gasoline) as a repr ...
Stoichiometry File
... our simple model does not show the full picture. What additional factors could we consider to get a more complete description of engine chemistry? We know that gasoline itself contains a wide array of hydrocarbons. So the presence of hydrocarbons in the exhaust most likely indicates that some of the ...
... our simple model does not show the full picture. What additional factors could we consider to get a more complete description of engine chemistry? We know that gasoline itself contains a wide array of hydrocarbons. So the presence of hydrocarbons in the exhaust most likely indicates that some of the ...
(III) From Aqueous Solutions with Sodium Dodecyl Sulfate
... concentrates using technology based on physic-chemical methods: extraction by organic reagents, ion exchange [4, 5]. It deals with the application of ion flotation with surfaceactive substances (surfactants) [6, 7]. Solvent sublation ( S S ) is the adsorptive bubble method for surface separation in ...
... concentrates using technology based on physic-chemical methods: extraction by organic reagents, ion exchange [4, 5]. It deals with the application of ion flotation with surfaceactive substances (surfactants) [6, 7]. Solvent sublation ( S S ) is the adsorptive bubble method for surface separation in ...
Chemical Bonding
... You will recall, from Chapter 1, that atomic theory describes electrons moving about the nucleus of the atom in energy levels, and that the electrons in the outermost energy level are called the valence electrons. It is the valence electrons of an atom that form chemical bonds. According to atomic t ...
... You will recall, from Chapter 1, that atomic theory describes electrons moving about the nucleus of the atom in energy levels, and that the electrons in the outermost energy level are called the valence electrons. It is the valence electrons of an atom that form chemical bonds. According to atomic t ...
ap chemistry 2005/2006
... Standard Enthalpies of Formation Present and future energy sources Lab: Observing Heat Changes (30 minutes) – mixing/observation of three thermochemical reactions, identification of endothermic or exothermic, identification as physical or chemical change. Lab: Determining the Specific Heat of an Unk ...
... Standard Enthalpies of Formation Present and future energy sources Lab: Observing Heat Changes (30 minutes) – mixing/observation of three thermochemical reactions, identification of endothermic or exothermic, identification as physical or chemical change. Lab: Determining the Specific Heat of an Unk ...
4U Chemistry Practice Exam - Coristines
... b. Zn2 c. H d. Cu2 e. Mg2 A voltaic cell has a solid lead electrode in a solution of 1.0 mol/L Pb(NO3)2 and a solid zinc electrode in a solution of 1.0 mol/L Zn(NO3)2. What reactant will be reduced? a. Zn2 b. Pb2 c. Pb d. Zn e. NO3 Which statement about electrochemical cells is not cor ...
... b. Zn2 c. H d. Cu2 e. Mg2 A voltaic cell has a solid lead electrode in a solution of 1.0 mol/L Pb(NO3)2 and a solid zinc electrode in a solution of 1.0 mol/L Zn(NO3)2. What reactant will be reduced? a. Zn2 b. Pb2 c. Pb d. Zn e. NO3 Which statement about electrochemical cells is not cor ...
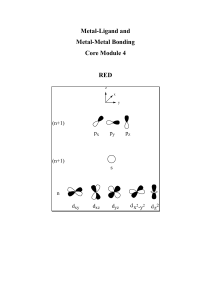
Metal-Ligand and Metal-Metal Bonding Lecture Notes
... use common nomenclature in transition metal chemistry. count valence electrons and determine metal oxidation state in transition metal complexes. Understand the physical basis of the 18-electron rule. appreciate the synergic nature of bonding in metal carbonyl complexes. understand the relationship ...
... use common nomenclature in transition metal chemistry. count valence electrons and determine metal oxidation state in transition metal complexes. Understand the physical basis of the 18-electron rule. appreciate the synergic nature of bonding in metal carbonyl complexes. understand the relationship ...
PHY 112 Master Syllabus
... • a broad view of the physics of electromagnetism, which is the combination of electric and magnetic phenomena, the basis of the natural world. • a knowledge of electric and magnetic quantities and physical laws associated with them. • a knowledge of the related mathematics required to manipulate th ...
... • a broad view of the physics of electromagnetism, which is the combination of electric and magnetic phenomena, the basis of the natural world. • a knowledge of electric and magnetic quantities and physical laws associated with them. • a knowledge of the related mathematics required to manipulate th ...
Regents Chemistry - New York Science Teacher
... substances have molecules that contain two carbon atoms, one oxygen atom, and six hydrogen atoms. These two substances must be ...
... substances have molecules that contain two carbon atoms, one oxygen atom, and six hydrogen atoms. These two substances must be ...
PHY 114 Master Syllabus
... include electric and magnetic fields, electric and magnetic flux, electric and magnetic dipoles, electric potential, and elementary circuits consisting of batteries, resistors, capacitors and inductors. Also studied are the physical laws associated with electromagnetic phenomena including Coulomb's ...
... include electric and magnetic fields, electric and magnetic flux, electric and magnetic dipoles, electric potential, and elementary circuits consisting of batteries, resistors, capacitors and inductors. Also studied are the physical laws associated with electromagnetic phenomena including Coulomb's ...
Equilibrium
... b. If raising the temperature of the reaction results in an equilibrium with a higher concentration of C than A, how will the value of Keq change? 12. The following reaction occurs when steam is passed over hot carbon. The mixture of gases it generates is called water gas and is useful as an indust ...
... b. If raising the temperature of the reaction results in an equilibrium with a higher concentration of C than A, how will the value of Keq change? 12. The following reaction occurs when steam is passed over hot carbon. The mixture of gases it generates is called water gas and is useful as an indust ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.