name - cloudfront.net
... 6. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them through oppositely ...
... 6. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them through oppositely ...
- Jersey College For Girls
... Q9. When lithium is burned in air, the two compounds lithium oxide (Li2O) and lithium nitride (Li3N) are formed. Both compounds are ionic and their ions can be represented by dot and cross diagrams. The dot and cross diagram for the ions in lithium oxide is ...
... Q9. When lithium is burned in air, the two compounds lithium oxide (Li2O) and lithium nitride (Li3N) are formed. Both compounds are ionic and their ions can be represented by dot and cross diagrams. The dot and cross diagram for the ions in lithium oxide is ...
final exam review chapter 1-4
... 5. If you have 4 g NaOH, and 10 g HBr, what is the limiting reagent and how much salt is produced? In lab if you produce1 g salt, what is the percent yield? ...
... 5. If you have 4 g NaOH, and 10 g HBr, what is the limiting reagent and how much salt is produced? In lab if you produce1 g salt, what is the percent yield? ...
AP Biology chap 2 HW - yhs
... ionic bonds--are strong only if the participating ions are hydrated b. hydrogen bonds--are responsible for bonding oxygen and hydrogen to form a single water molecule c. polar covalent bonds--can occur between two atoms of the same element d. covalent bonds--may be single, double, or triple e. hydro ...
... ionic bonds--are strong only if the participating ions are hydrated b. hydrogen bonds--are responsible for bonding oxygen and hydrogen to form a single water molecule c. polar covalent bonds--can occur between two atoms of the same element d. covalent bonds--may be single, double, or triple e. hydro ...
circuits 1.notebook
... Circuit- any path in which electrons flow series - a circuit in which there is only one path that the electrons can travel ****a break in the series circuit causes all current to stop**** parallel- the devices are connected to the same two points of an electrical circuit providing more than one path ...
... Circuit- any path in which electrons flow series - a circuit in which there is only one path that the electrons can travel ****a break in the series circuit causes all current to stop**** parallel- the devices are connected to the same two points of an electrical circuit providing more than one path ...
C2 revision slides V3 + questions + MS
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
Chapter 4 - Reactions in Aqueous Solutions
... base, or both. (a) HI, (b) CH3COO-, (c) H2PO4HI (aq) ...
... base, or both. (a) HI, (b) CH3COO-, (c) H2PO4HI (aq) ...
File
... example of each. 23. (a) Define coagulation or flocculation. (b) What is the difference between Lyophilic and Lyophobic colloids? 24. What are proteins? Describe simple and conjugated protein with at least one example in each case. 25. (a) Amines have lower boiling points than corresponding alcohols ...
... example of each. 23. (a) Define coagulation or flocculation. (b) What is the difference between Lyophilic and Lyophobic colloids? 24. What are proteins? Describe simple and conjugated protein with at least one example in each case. 25. (a) Amines have lower boiling points than corresponding alcohols ...
Practice Exam 2 - Department of Chemistry and Biochemistry
... A) atoms are held together by sharing electrons. B) oppositely charged ions are held together by strong electrical attractions. C) atoms of different metals form bonds. D) atoms of noble gases are held together by attractions between oppositely charged ions. E) atoms of metals form bonds to atoms of ...
... A) atoms are held together by sharing electrons. B) oppositely charged ions are held together by strong electrical attractions. C) atoms of different metals form bonds. D) atoms of noble gases are held together by attractions between oppositely charged ions. E) atoms of metals form bonds to atoms of ...
Unit 1 - Learning Objectives
... There are many everyday examples of uses of catalysts. Catalysts are used in many industrial processes. Heterogeneous catalysts work by the adsorption of reactant molecules. The surface activity of a catalyst can be reduced by poisoning. Impurities in the reactants result in industrial cat ...
... There are many everyday examples of uses of catalysts. Catalysts are used in many industrial processes. Heterogeneous catalysts work by the adsorption of reactant molecules. The surface activity of a catalyst can be reduced by poisoning. Impurities in the reactants result in industrial cat ...
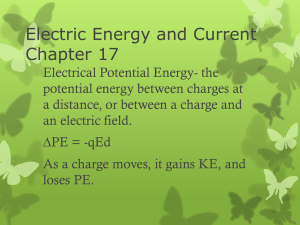
Electric Energy and Current Chapter 17
... something between the plates of a capacitor. We call this a dielectric. A dielectric is an insulating materialexamples are glass, rubber, wood, waxed paper, etc. Molecules in dielectric become polarized, line up with electric field. This allows for a weaker electric field between the plates, so the ...
... something between the plates of a capacitor. We call this a dielectric. A dielectric is an insulating materialexamples are glass, rubber, wood, waxed paper, etc. Molecules in dielectric become polarized, line up with electric field. This allows for a weaker electric field between the plates, so the ...
AP Chemistry 2013 Semester 1 Final Exam Review Problems
... 14.52% C, 1.83% H, 64.30% Cl and 19.35% O by mass and has a molar mass of 165.4g/mol. a. What is the empirical formula of this substance? b. What is the molecular formula of this substance? c. Draw the Lewis structure of the molecule using the fact that the Cl atoms bond to a single C atom, there is ...
... 14.52% C, 1.83% H, 64.30% Cl and 19.35% O by mass and has a molar mass of 165.4g/mol. a. What is the empirical formula of this substance? b. What is the molecular formula of this substance? c. Draw the Lewis structure of the molecule using the fact that the Cl atoms bond to a single C atom, there is ...
Int. to Basic Electronics - Kashif Bashir
... electron(-ve charge) and proton(+ve charge). • Separate and opposite charges at the two terminals, electric energy can be supplied to a circuit connected to the battery. • An atom is the smallest particle of the basic elements that form solid,liquids, and gases we know as physical substances. • In a ...
... electron(-ve charge) and proton(+ve charge). • Separate and opposite charges at the two terminals, electric energy can be supplied to a circuit connected to the battery. • An atom is the smallest particle of the basic elements that form solid,liquids, and gases we know as physical substances. • In a ...
2 (aq)
... Combustion Reactions • Is a chemical change in which an element or a compound reacts with oxygen often producing energy of the form of heat and light – Examples: 2C8H16(l) + 25O2(g) 16CO2(g) + 18H2O(l) 2Mg(s) + O2(g) 2MgO(s) S(s) + O2(g) SO2(g) ...
... Combustion Reactions • Is a chemical change in which an element or a compound reacts with oxygen often producing energy of the form of heat and light – Examples: 2C8H16(l) + 25O2(g) 16CO2(g) + 18H2O(l) 2Mg(s) + O2(g) 2MgO(s) S(s) + O2(g) SO2(g) ...
Chemical Reactions: Introduction to Reaction Types
... solid, (s). For a precipitation reaction to occur, at least one of the products must be insoluble; if both products are soluble, then no reaction occurs. The presence of a precipitate is observed in the lab as a cloudy mixture that results when two solutions are mixed. The following is an example of ...
... solid, (s). For a precipitation reaction to occur, at least one of the products must be insoluble; if both products are soluble, then no reaction occurs. The presence of a precipitate is observed in the lab as a cloudy mixture that results when two solutions are mixed. The following is an example of ...
Click to download. - Life Learning Cloud
... The atoms may be the same (e.g. O2) or different (e.g. H2O). The chemical formula shows the number and type of atoms present. Non-metal compounds are made of molecules: Carbon dioxide contains CO2 molecules Methane (natural gas) contains CH4 molecules AN ION is an atom or group of atoms with an elec ...
... The atoms may be the same (e.g. O2) or different (e.g. H2O). The chemical formula shows the number and type of atoms present. Non-metal compounds are made of molecules: Carbon dioxide contains CO2 molecules Methane (natural gas) contains CH4 molecules AN ION is an atom or group of atoms with an elec ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.




![C2_revision_slides_V3_+_questions_+_MS_-_H[1]](http://s1.studyres.com/store/data/000092833_1-97fb33725e7f1ef12029ed42751d3dca-300x300.png)