synthesis and reactions of tris dialkyl dithiocarbamates of group 15
... spectroscopies. Although the known structures of these complexes are apparently quite varied, their spectra can be explained in terms of a common centrosymmetric halogen–bridged dimer. This demonstrates obviously the risk of structural elucidation from spectroscopic data alone. Correlations between ...
... spectroscopies. Although the known structures of these complexes are apparently quite varied, their spectra can be explained in terms of a common centrosymmetric halogen–bridged dimer. This demonstrates obviously the risk of structural elucidation from spectroscopic data alone. Correlations between ...
Ch 21 - Keene ISD
... is possible to store potential energy in a system of interacting objects. In this chapter, we’ll learn about a new form of potential energy, electric potential energy. • This roller coaster is pulled to the top of the first hill by a chain. The tension in the chain does work on the coaster, increasi ...
... is possible to store potential energy in a system of interacting objects. In this chapter, we’ll learn about a new form of potential energy, electric potential energy. • This roller coaster is pulled to the top of the first hill by a chain. The tension in the chain does work on the coaster, increasi ...
Document
... is possible to store potential energy in a system of interacting objects. In this chapter, we’ll learn about a new form of potential energy, electric potential energy. • This roller coaster is pulled to the top of the first hill by a chain. The tension in the chain does work on the coaster, increasi ...
... is possible to store potential energy in a system of interacting objects. In this chapter, we’ll learn about a new form of potential energy, electric potential energy. • This roller coaster is pulled to the top of the first hill by a chain. The tension in the chain does work on the coaster, increasi ...
National German Competition and Problems of the IChO
... b) Of which volume does the reactor have to be if 29 t of ethyl acetate shall be produced during one day. Between two production cycles additional 25 minutes are needed for discharging, cleaning and refilling the reactor. Note to b): 1. To solve the problem the solution of the integral below is need ...
... b) Of which volume does the reactor have to be if 29 t of ethyl acetate shall be produced during one day. Between two production cycles additional 25 minutes are needed for discharging, cleaning and refilling the reactor. Note to b): 1. To solve the problem the solution of the integral below is need ...
SCH4U TEXT BOOK
... How do you predict whether or not a molecule that contains polar bonds has an overall molecular polarity? To determine molecular polarity, you must consider the shape of the molecule and the bond dipoles within the molecule. If equal bond dipoles act in opposite directions in three-dimensional space ...
... How do you predict whether or not a molecule that contains polar bonds has an overall molecular polarity? To determine molecular polarity, you must consider the shape of the molecule and the bond dipoles within the molecule. If equal bond dipoles act in opposite directions in three-dimensional space ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concentration of all the ions left in solution? 3) When 100 mL of 0.40M NaOH is added to 75 mL of 0.6 M ...
... 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concentration of all the ions left in solution? 3) When 100 mL of 0.40M NaOH is added to 75 mL of 0.6 M ...
MAJOR - Bijni College
... Debye-Huckel-Onsager equation, Stokes-Einstein relation. Activity of ions. DebyeHuckel theory (elementary ideas) of strong electrolytes. Ionic strength of solutions. Electrochemical cells, measurement of emf, electrode potenmtial, sign convention. Different types of electrodes, the calomel electrode ...
... Debye-Huckel-Onsager equation, Stokes-Einstein relation. Activity of ions. DebyeHuckel theory (elementary ideas) of strong electrolytes. Ionic strength of solutions. Electrochemical cells, measurement of emf, electrode potenmtial, sign convention. Different types of electrodes, the calomel electrode ...
cmc by uv 2
... of ionic surfactants. The change in the electrical conductance of aqueous ionic surfactant solutions at the CMC is due to the different degree of surfactant ionization below (surfactant monomers behave as strong electrolytes) and above (micelles are partially ionized) the CMC. On the assumption that ...
... of ionic surfactants. The change in the electrical conductance of aqueous ionic surfactant solutions at the CMC is due to the different degree of surfactant ionization below (surfactant monomers behave as strong electrolytes) and above (micelles are partially ionized) the CMC. On the assumption that ...
Acid-Base Biochemistry
... a base; thus, AlCl3, BF3, and SO3 are acids. ► The Lewis theory defines an acid as a species that can accept an electron pair from another atom, and a base as a species that can donate an electron pair to complete the valence shell of ...
... a base; thus, AlCl3, BF3, and SO3 are acids. ► The Lewis theory defines an acid as a species that can accept an electron pair from another atom, and a base as a species that can donate an electron pair to complete the valence shell of ...
m - DepositOnce
... 2.3. Physical Properties of the Vanadium Oxides In this chapter the structur ...
... 2.3. Physical Properties of the Vanadium Oxides In this chapter the structur ...
Organic Reactions in Organised Media
... pore openings. The approach offers possibilities for reuse of the material since the particles can easily be removed from the reaction mixture by filtration or by centrifugation. Another potential use of mesoporous materials in preparative organic chemistry is for homogeneous catalysis. The catalyst ...
... pore openings. The approach offers possibilities for reuse of the material since the particles can easily be removed from the reaction mixture by filtration or by centrifugation. Another potential use of mesoporous materials in preparative organic chemistry is for homogeneous catalysis. The catalyst ...
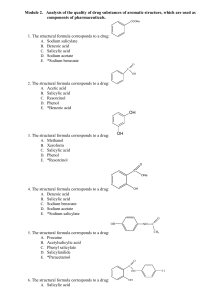
Module 2. Drug substances of aromatic structure
... D. Phenolphthalein E. *Solution of starch 81. The chemical name of xeroform: A. Oxybenzene B. Dihydroxybenzene C. 3-Methyl-5-methylphenol D. 5-Methyl-2-(methylethyl)phenol E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. ...
... D. Phenolphthalein E. *Solution of starch 81. The chemical name of xeroform: A. Oxybenzene B. Dihydroxybenzene C. 3-Methyl-5-methylphenol D. 5-Methyl-2-(methylethyl)phenol E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. ...
Energy Changes in Chemical Reactions
... 10.2 Introduction to Thermodynamics Thermochemistry is part of a broader subject called thermodynamics, which is the scientific study of the interconversion of heat and other kinds of energy. The laws of thermodynamics provide useful guidelines for understanding the energetics and directions of pr ...
... 10.2 Introduction to Thermodynamics Thermochemistry is part of a broader subject called thermodynamics, which is the scientific study of the interconversion of heat and other kinds of energy. The laws of thermodynamics provide useful guidelines for understanding the energetics and directions of pr ...
Specification – AS/A Level Chemistry A
... Raw materials are converted into new and useful substances by chemical reactions. The theoretical yield of a chemical reaction can be calculated. 1.1 Module 1: Atoms and Reactions This module provides candidates with a knowledge and understanding of atomic structure and the chemical ideas that under ...
... Raw materials are converted into new and useful substances by chemical reactions. The theoretical yield of a chemical reaction can be calculated. 1.1 Module 1: Atoms and Reactions This module provides candidates with a knowledge and understanding of atomic structure and the chemical ideas that under ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.