08272012BC Science Chem 12 Chapter 1 Answer Key
... water will occur as water is formed in a reaction that occurs in aqueous solution. This is, of course, nonsense! As the entire reaction occurs in the solvent water, there will simply be a small amount of water formed, replacing the hydrogen and oxygen atoms (actually hydrogen and hydroxide ions) rea ...
... water will occur as water is formed in a reaction that occurs in aqueous solution. This is, of course, nonsense! As the entire reaction occurs in the solvent water, there will simply be a small amount of water formed, replacing the hydrogen and oxygen atoms (actually hydrogen and hydroxide ions) rea ...
Chemical Equilibrium
... This is simply the reaction between elemental hydrogen and elemental iodine to make hydrogen iodide. The way the equation is written, we are led to believe that the reaction goes to completion, that all the H2 and the I2 react to make HI. However, this is not the case. The reverse chemical reaction ...
... This is simply the reaction between elemental hydrogen and elemental iodine to make hydrogen iodide. The way the equation is written, we are led to believe that the reaction goes to completion, that all the H2 and the I2 react to make HI. However, this is not the case. The reverse chemical reaction ...
National German Competition
... q) Write down the equation of the reaction of compound 1 with lithium dimethylcuprate and water. Give the complete names of the alcohols. Zinc organic compounds are longer known and more often used. These compounds are applied to synthesize alcohols, more exactly in the synthesis of hydroxy esters. ...
... q) Write down the equation of the reaction of compound 1 with lithium dimethylcuprate and water. Give the complete names of the alcohols. Zinc organic compounds are longer known and more often used. These compounds are applied to synthesize alcohols, more exactly in the synthesis of hydroxy esters. ...
Instructor`s Guide to General Chemistry: Guided
... of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be found. Molecules/ions react and molecules/ions are produced, so the units to keep tr ...
... of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be found. Molecules/ions react and molecules/ions are produced, so the units to keep tr ...
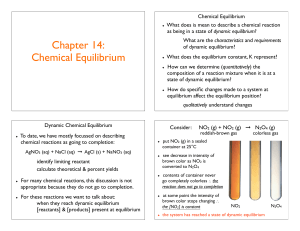
Chapter 14: Chemical Equilibrium
... as being in a state of dynamic equilibrium? What are the characteristics and requirements of dynamic equilibrium? ...
... as being in a state of dynamic equilibrium? What are the characteristics and requirements of dynamic equilibrium? ...
Physical Sciences Grade 10 Term 2
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
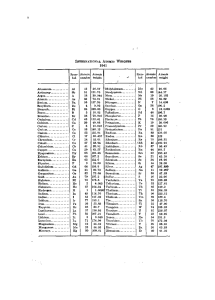
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... of the student. When an experiment is too short, the student will find interesting things to do under "Suggestions for Further Work"; when it is too long, the instructor will select parts of the "Procedure" which may be omitted. Several of the procedures have been expanded and the directions made mo ...
... of the student. When an experiment is too short, the student will find interesting things to do under "Suggestions for Further Work"; when it is too long, the instructor will select parts of the "Procedure" which may be omitted. Several of the procedures have been expanded and the directions made mo ...
Chemical Quantities
... To understand the molecular and mass information given in a balanced equation. Reactions are what chemistry is really all about. Recall from Chapter 6 that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the ...
... To understand the molecular and mass information given in a balanced equation. Reactions are what chemistry is really all about. Recall from Chapter 6 that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the ...
chemistry - Textbooks Online
... Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry studies the reactions of carbon compounds, which are 100 times more numerous than nonorganic ones. It also studies an immense variety of molecules, incl ...
... Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry studies the reactions of carbon compounds, which are 100 times more numerous than nonorganic ones. It also studies an immense variety of molecules, incl ...
Chemical Equilibria - Beck-Shop
... reaction will occur. The rate of the forward reaction (determined by the gradient of the tangent drawn to the concentration versus time plot) is at its peak since [reactants] is at its highest while the rate of the backward reaction is zero. As the reaction progresses, the rate of the forward reacti ...
... reaction will occur. The rate of the forward reaction (determined by the gradient of the tangent drawn to the concentration versus time plot) is at its peak since [reactants] is at its highest while the rate of the backward reaction is zero. As the reaction progresses, the rate of the forward reacti ...
Amines - ncert
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
equilibrium - eVirtualGuru
... When a liquid evaporates in a closed container, molecules with relatively higher kinetic energy escape the liquid surface into the vapour phase and number of liquid molecules from the vapour phase strike the liquid surface and are retained in the liquid phase. It gives rise to a constant vapour pres ...
... When a liquid evaporates in a closed container, molecules with relatively higher kinetic energy escape the liquid surface into the vapour phase and number of liquid molecules from the vapour phase strike the liquid surface and are retained in the liquid phase. It gives rise to a constant vapour pres ...
IIT-JEE (Advanced) - Brilliant Public School Sitamarhi
... If the amount of O2 evolved was 146.8 ml at S.T.P., calculate the % by weight of KClO4 in the residue. Q.13 A sample of calcium carbonate contains impurities which do not react with a mineral acid. When 2 grams of the sample were reacted with the mineral acid, 375 ml of carbon dioxide were obtained ...
... If the amount of O2 evolved was 146.8 ml at S.T.P., calculate the % by weight of KClO4 in the residue. Q.13 A sample of calcium carbonate contains impurities which do not react with a mineral acid. When 2 grams of the sample were reacted with the mineral acid, 375 ml of carbon dioxide were obtained ...
Equilibrium
... The value of the equilibrium constant for any reaction can be determined by experiment. As detailed in the above section, the equilibrium position for a given reaction does not depend on the starting concentrations, so the equilibrium constant has the same value regardless of the initial amounts of ...
... The value of the equilibrium constant for any reaction can be determined by experiment. As detailed in the above section, the equilibrium position for a given reaction does not depend on the starting concentrations, so the equilibrium constant has the same value regardless of the initial amounts of ...
Support Material
... Q.28. Classify each of the following as either a p-type or n-type semi-conductor : (a) Ge doped with In (b) B doped with Si Ans. Hint : (a) Ge is group 14 element and In is group 13 element. Therefore, an electron de cit hole is created. Thus semi-conductor is p-type. (b) Since B is group 13 element ...
... Q.28. Classify each of the following as either a p-type or n-type semi-conductor : (a) Ge doped with In (b) B doped with Si Ans. Hint : (a) Ge is group 14 element and In is group 13 element. Therefore, an electron de cit hole is created. Thus semi-conductor is p-type. (b) Since B is group 13 element ...
Stoichiometry
... hydrogen gas to form solid copper and liquid water. CuO (s) + H2 (g) ---> Cu (s) + H2O (l) • Aluminum metal reacts with oxygen gas to form solid aluminum oxide. Al (s) + O2 (g) ---> Al2O3 (s) Stoichiometry ...
... hydrogen gas to form solid copper and liquid water. CuO (s) + H2 (g) ---> Cu (s) + H2O (l) • Aluminum metal reacts with oxygen gas to form solid aluminum oxide. Al (s) + O2 (g) ---> Al2O3 (s) Stoichiometry ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.