ELECTROCHEMICAL STUDY OF CORROSION PROCESSES IN
... processes in high temperature aqueous systems, depending upon the density and dielectric constant of the systems. EO corrosion processes are featured by partial charge transfer reactions such as the metal dissolution and the reduction of oxygen. On the other hand, CO processes are dominated by direc ...
... processes in high temperature aqueous systems, depending upon the density and dielectric constant of the systems. EO corrosion processes are featured by partial charge transfer reactions such as the metal dissolution and the reduction of oxygen. On the other hand, CO processes are dominated by direc ...
step by step Stoichiometry
... Or 80.3 divided by 55.847, multiplied by 3, divided by 2, multiplied by 28.01015 ...
... Or 80.3 divided by 55.847, multiplied by 3, divided by 2, multiplied by 28.01015 ...
From Kinetics to Equilibrium
... acing cars can reach speeds that are well above 200 km/h. In contrast, the maximum speed of many farm tractors is only about 25 km/h. Just as some vehicles travel more quickly than others, some chemical reactions occur more quickly than others. For example, compare the two reactions that occur in ve ...
... acing cars can reach speeds that are well above 200 km/h. In contrast, the maximum speed of many farm tractors is only about 25 km/h. Just as some vehicles travel more quickly than others, some chemical reactions occur more quickly than others. For example, compare the two reactions that occur in ve ...
Syllabus Cambridge International A & AS Level Chemistry Syllabus code 9701
... To be considered for an AICE Diploma, a candidate must earn the equivalent of six credits by passing a combination of examinations at either double credit or single credit, with at least one course coming from each of the three curriculum areas. The examinations are administered in May/June and Octo ...
... To be considered for an AICE Diploma, a candidate must earn the equivalent of six credits by passing a combination of examinations at either double credit or single credit, with at least one course coming from each of the three curriculum areas. The examinations are administered in May/June and Octo ...
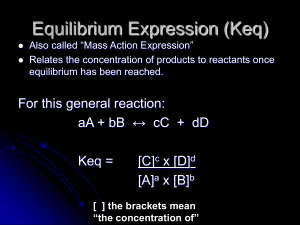
Equilibrium Expression (Keq)
... If the Ksp of RaSO4 = 4 x 10-11 calculate its solubility in pure water. RaSO4 (s) ↔ Ra+2 (aq) + SO4-2 (aq) Ksp = [Ra+2] x [SO4-2] ...
... If the Ksp of RaSO4 = 4 x 10-11 calculate its solubility in pure water. RaSO4 (s) ↔ Ra+2 (aq) + SO4-2 (aq) Ksp = [Ra+2] x [SO4-2] ...
Inorganic Chemistry
... before Chapters 5 or 6. Chapter 6 contains material dealing with intermolecular forces and polarity of molecules because of the importance of these topics when interpreting properties of substances and their chemical behavior. In view of the importance of the topic, especially in industrial chemistr ...
... before Chapters 5 or 6. Chapter 6 contains material dealing with intermolecular forces and polarity of molecules because of the importance of these topics when interpreting properties of substances and their chemical behavior. In view of the importance of the topic, especially in industrial chemistr ...
The role of formic acid pretreatment in improving the carboxyl
... acid concentration increases. But, hemicellulose, lignin and cellulose fragments are much more likely to dissolve when using a higher concentration of formic acid solution. Therefore, it seems like fines are reduced. In Figure 2 (e), the fibers were oxidized with TEMPO. It can be seen that the long ...
... acid concentration increases. But, hemicellulose, lignin and cellulose fragments are much more likely to dissolve when using a higher concentration of formic acid solution. Therefore, it seems like fines are reduced. In Figure 2 (e), the fibers were oxidized with TEMPO. It can be seen that the long ...
Study Guide for Content Mastery - Student Edition
... has six study guide pages of questions and exercises for you to complete as you read the text. The study guide pages are divided into sections that match those in your text. You will find that the directions in the Study Guide for Content Mastery are simply stated and easy to follow. Sometimes you w ...
... has six study guide pages of questions and exercises for you to complete as you read the text. The study guide pages are divided into sections that match those in your text. You will find that the directions in the Study Guide for Content Mastery are simply stated and easy to follow. Sometimes you w ...
105 ACID - DW Brooks
... Hydrangea is a shrub with clusters of showy flowers. The color of its flowers can change as the pH ofthe soil in which it grows changes. The flowers are red-pink under neutral to low soil acidity and blue under conditions of higher acidity. Hydrangeas contain a natural indicator, anthocyanin, which ...
... Hydrangea is a shrub with clusters of showy flowers. The color of its flowers can change as the pH ofthe soil in which it grows changes. The flowers are red-pink under neutral to low soil acidity and blue under conditions of higher acidity. Hydrangeas contain a natural indicator, anthocyanin, which ...
Water Chemistry - U
... systems (e.g., water and wastewater treatment systems and hazardous waste processing). Most existing textbooks also focus on solving inorganic ionic equilibria using graphical and manual algebraic approaches, and with a few exceptions, they do not focus on the use of computer programs to solve probl ...
... systems (e.g., water and wastewater treatment systems and hazardous waste processing). Most existing textbooks also focus on solving inorganic ionic equilibria using graphical and manual algebraic approaches, and with a few exceptions, they do not focus on the use of computer programs to solve probl ...
PHOSPHORUS AND SULFUR COSMOCHEMISTRY
... Phosphorus is a key element for life. This work reviews the role of phosphorus in life. Theories on the origin of life are confounded by a lack of reactive phosphorus, and attempts to overcome the dearth of reactive phosphorus must employ unrealistic phosphorus compounds, energetic organic compounds ...
... Phosphorus is a key element for life. This work reviews the role of phosphorus in life. Theories on the origin of life are confounded by a lack of reactive phosphorus, and attempts to overcome the dearth of reactive phosphorus must employ unrealistic phosphorus compounds, energetic organic compounds ...
14.1 Dynamic Equilibrium, Keq , and the Mass Action Expression
... The reaction coordinate diagram shows how the concentration of N2O4 and NO2 changes as the reaction approaches equilibrium. This is also reflected in Q. As the reaction proceeds to the right, N2O4 to NO2, the Q value increases, N2O4 becomes smaller and NO2 becomes larger. The reaction finally reache ...
... The reaction coordinate diagram shows how the concentration of N2O4 and NO2 changes as the reaction approaches equilibrium. This is also reflected in Q. As the reaction proceeds to the right, N2O4 to NO2, the Q value increases, N2O4 becomes smaller and NO2 becomes larger. The reaction finally reache ...
Slide 1
... • The equilibrium constant, Keq, is the numerical value of the ratio of product concentrations to reactant concentrations, with each concentration raised to the power corresponding to its coefficient in the balanced equation. • The value of Keq is constant only at a ...
... • The equilibrium constant, Keq, is the numerical value of the ratio of product concentrations to reactant concentrations, with each concentration raised to the power corresponding to its coefficient in the balanced equation. • The value of Keq is constant only at a ...
Hardness - ChemGod.com
... Ca2+(aq)+ 2 HCO3-(aq) → CaCO3 (s) + CO2 (g) + H2O(l) Bicarbonate hardness in the presence of softeners!: Ca2+(aq)+ 2 HCO3-(aq) + Ca(OH)2 (s) → 2 CaCO3 (s) + 2 H2O(l) ...
... Ca2+(aq)+ 2 HCO3-(aq) → CaCO3 (s) + CO2 (g) + H2O(l) Bicarbonate hardness in the presence of softeners!: Ca2+(aq)+ 2 HCO3-(aq) + Ca(OH)2 (s) → 2 CaCO3 (s) + 2 H2O(l) ...
Chapter One Hemilabile Ligands in Transition
... hemilabile ligands. For example, a Cu(I) based catalyst will bind quite strongly to a soft donor atom, but during a catalytic process will be oxidised to Cu(II), changing its binding properties significantly. The slight increase in hardness of the Cu(II) ...
... hemilabile ligands. For example, a Cu(I) based catalyst will bind quite strongly to a soft donor atom, but during a catalytic process will be oxidised to Cu(II), changing its binding properties significantly. The slight increase in hardness of the Cu(II) ...
Solutions for Chapter 8 End-of-Chapter Problems
... The conclusion in Problem 8.10(c) is reinforced by these results: the more solute molecules in a given volume, the greater the number of arrangements. The same restriction applies here. To see the affect on W when n > N/2, calculate W for N = 20 and n = 9 and 11. Is the result at all surprising? Why ...
... The conclusion in Problem 8.10(c) is reinforced by these results: the more solute molecules in a given volume, the greater the number of arrangements. The same restriction applies here. To see the affect on W when n > N/2, calculate W for N = 20 and n = 9 and 11. Is the result at all surprising? Why ...
CBSE (Mains)
... (1) Small animals like rats will die after drinking river water (2) The increased microbial activity releases micro-nutrients such as iron (3) The increased microbial activity uses up dissolved oxygen (4) The river water is still suitable for drinking as impurities are only about 0.1% Sol: Ans [3] ...
... (1) Small animals like rats will die after drinking river water (2) The increased microbial activity releases micro-nutrients such as iron (3) The increased microbial activity uses up dissolved oxygen (4) The river water is still suitable for drinking as impurities are only about 0.1% Sol: Ans [3] ...
Solving General Chemistry Problems 5e
... straightforward way to solve that type. Recognition of the problem (not the mechanics of the solution) often is the biggest difficulty to be overcome. In most cases, there are only a few types of problems associated with a given topic. 8. Be sure that you understand material, rather than just being ...
... straightforward way to solve that type. Recognition of the problem (not the mechanics of the solution) often is the biggest difficulty to be overcome. In most cases, there are only a few types of problems associated with a given topic. 8. Be sure that you understand material, rather than just being ...
Chemsheets AS 1027
... a) reaction of hydrochloric acid (aq) with potassium hydroxide (aq) b) precipitation of silver iodide from reaction between silver nitrate (aq) and potassium iodide (aq) c) reaction of potassium carbonate (aq) with nitric acid (aq) d) precipitation of calcium hydroxide from reaction between sodium h ...
... a) reaction of hydrochloric acid (aq) with potassium hydroxide (aq) b) precipitation of silver iodide from reaction between silver nitrate (aq) and potassium iodide (aq) c) reaction of potassium carbonate (aq) with nitric acid (aq) d) precipitation of calcium hydroxide from reaction between sodium h ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.