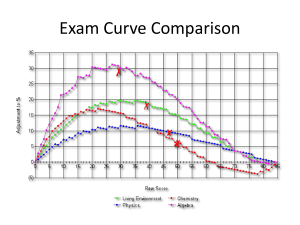
Abdullah F. Eid
... The octahedra are arranged in four M3O13 groups. Each group is formed by three edges sharing octahedra and having a common oxygen atom which is also shared with the central tetrahedron XO4 . Among a wide variety of HPAs, the Keggin’s are the most stable and more easily available; these are the most ...
... The octahedra are arranged in four M3O13 groups. Each group is formed by three edges sharing octahedra and having a common oxygen atom which is also shared with the central tetrahedron XO4 . Among a wide variety of HPAs, the Keggin’s are the most stable and more easily available; these are the most ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 39 ...
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 39 ...
TRO Chapter 4
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 39 ...
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 39 ...
Document
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 39 ...
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 39 ...
AP Chemistry - Siva Kodali
... 2,000 students per year. She is the author of ACT For Dummies, Pre-Calculus For Dummies, AP Biology For Dummies, Chemistry Workbook For Dummies, GRE For Dummies, Pre-Calculus Workbook for Dummies, and other books on self-esteem, writing, and motivational topics. Michelle has overseen dozens of progr ...
... 2,000 students per year. She is the author of ACT For Dummies, Pre-Calculus For Dummies, AP Biology For Dummies, Chemistry Workbook For Dummies, GRE For Dummies, Pre-Calculus Workbook for Dummies, and other books on self-esteem, writing, and motivational topics. Michelle has overseen dozens of progr ...
Unit 6 Review Answers
... 0.1 mol/L CH3COOH because the HCl is completely ionized whereas the CH3COOH is only partly ionized. For solutions of these two acids at a concentration of 1 × 10–7 mol/L, the HCl is still ionized completely and the CH3COOH again is only partly ionized. However at such a low concentration the conduct ...
... 0.1 mol/L CH3COOH because the HCl is completely ionized whereas the CH3COOH is only partly ionized. For solutions of these two acids at a concentration of 1 × 10–7 mol/L, the HCl is still ionized completely and the CH3COOH again is only partly ionized. However at such a low concentration the conduct ...
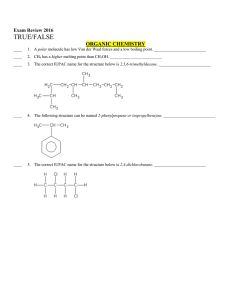
Multiple Choice Exam Review June 2016
... ____ 53. Conjugate acid base partners differ by a single proton. _________________________ ____ 54. Salts containing an anion which is found in a strong acid cannot act like weak bases. _________________________ ____ 55. If the pH of an aquatic solution at 95oC is 4.5 the pOH must be 9.5. __________ ...
... ____ 53. Conjugate acid base partners differ by a single proton. _________________________ ____ 54. Salts containing an anion which is found in a strong acid cannot act like weak bases. _________________________ ____ 55. If the pH of an aquatic solution at 95oC is 4.5 the pOH must be 9.5. __________ ...
Chapter 1 – Reaction Kinetics Answer Key
... (the quotient of moles/volume) does not change. For aqueous species, increased numbers of moles in the same volume of solution certainly does increase the concentration. The same is true for gases ...
... (the quotient of moles/volume) does not change. For aqueous species, increased numbers of moles in the same volume of solution certainly does increase the concentration. The same is true for gases ...
Daily Log
... an element, why isn’t it a mixture? Homework (3 sentences) – Propose a method to separate oil and water. Give me a property used to separate them and your process. ...
... an element, why isn’t it a mixture? Homework (3 sentences) – Propose a method to separate oil and water. Give me a property used to separate them and your process. ...
Exam Review
... c) maximum number of outer electrons b) maximum number of nucleons d) highest energy level ...
... c) maximum number of outer electrons b) maximum number of nucleons d) highest energy level ...
Topic 8 Acids and Bases File
... 8.1.1 Define acids and bases according to the Bronsted-Lowry and Lewis theories. .........................6 Reactions of Acids ..................................................................................................................................................9 8.1.2 Deduce whether or n ...
... 8.1.1 Define acids and bases according to the Bronsted-Lowry and Lewis theories. .........................6 Reactions of Acids ..................................................................................................................................................9 8.1.2 Deduce whether or n ...
Chapter 5 Geochemical Weathering
... Weathering of landscapes involves an array of mechanical and geochemical agents that conspire to alter primary geological formations to sediments and solutes. Geochemical weathering is driven by water. In soils, water is the limiting factor for the activity of aerobic bacteria that degrade organics ...
... Weathering of landscapes involves an array of mechanical and geochemical agents that conspire to alter primary geological formations to sediments and solutes. Geochemical weathering is driven by water. In soils, water is the limiting factor for the activity of aerobic bacteria that degrade organics ...
Volumetrie properties of concentrated electrolyte solutions
... Z. KODEJŠ and P. PACÁK Institute of Inorganic Chemistry, Czechoslovak Academy of Sciences, CS-160 00 Prague ...
... Z. KODEJŠ and P. PACÁK Institute of Inorganic Chemistry, Czechoslovak Academy of Sciences, CS-160 00 Prague ...
Modern Chemistry
... 1. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model. a. A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density ...
... 1. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model. a. A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density ...
Types of Chemical Reactions
... Ions in Aqueous Solution Molecular and Ionic Equations • An total ionic equation, however, represents strong electrolytes as separate independent ions. This is a more accurate representation of the way electrolytes behave in solution. – A complete ionic equation is a chemical equation in which stro ...
... Ions in Aqueous Solution Molecular and Ionic Equations • An total ionic equation, however, represents strong electrolytes as separate independent ions. This is a more accurate representation of the way electrolytes behave in solution. – A complete ionic equation is a chemical equation in which stro ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... • other ionic compounds, like AgCl, dissolve hardly at all in water at room temperature • compounds that dissolve in a solvent are said to be soluble, while those that do not are said to be insoluble – NaCl is soluble in water, AgCl is insoluble in water – the degree of solubility depends on the tem ...
... • other ionic compounds, like AgCl, dissolve hardly at all in water at room temperature • compounds that dissolve in a solvent are said to be soluble, while those that do not are said to be insoluble – NaCl is soluble in water, AgCl is insoluble in water – the degree of solubility depends on the tem ...
Oxidative Alihatic Carbon-Carbon Bond Cleavage Reactions
... Rather, ferrous ion-mediated hydration of a vicinal triketone intermediate was the key factor in determining the regioselectivity of the C-C cleavage reaction. We have developed a high-yielding synthetic route to protected precursors of C(1)H acireductones. Preparation of the complexes [(6Ph2TPA)M(P ...
... Rather, ferrous ion-mediated hydration of a vicinal triketone intermediate was the key factor in determining the regioselectivity of the C-C cleavage reaction. We have developed a high-yielding synthetic route to protected precursors of C(1)H acireductones. Preparation of the complexes [(6Ph2TPA)M(P ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 40 ...
... surrounded by water molecules, insulating it from other ions The result is a solution with free moving charged particles able to conduct electricity 40 ...
Descriptive Inorganic Chemistry
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.