Final Exam (5/15/14)
... 9. In some bacteria, the citric acid cycle runs backwards from oxaloacetate to citrate to reduce CO2. We feed bacteria with oxaloacetate labeled with14-C on the methyl carbon (-CH2-). a. Draw the citrate molecule indicating where the label is. If only a fraction of molecules contain that label, indi ...
... 9. In some bacteria, the citric acid cycle runs backwards from oxaloacetate to citrate to reduce CO2. We feed bacteria with oxaloacetate labeled with14-C on the methyl carbon (-CH2-). a. Draw the citrate molecule indicating where the label is. If only a fraction of molecules contain that label, indi ...
Measuring Photosynthesis to Evaluate Photoprotection by
... Anthocyanin, like chlorophyll, is a pigment molecule present in plants. However, while chlorophyll reflects green light, anthocyanin reflects red light, a much lower frequency, and therefore absorbs less energy. Pigment molecules are very involved in photosynthesis in that they absorb the energy nee ...
... Anthocyanin, like chlorophyll, is a pigment molecule present in plants. However, while chlorophyll reflects green light, anthocyanin reflects red light, a much lower frequency, and therefore absorbs less energy. Pigment molecules are very involved in photosynthesis in that they absorb the energy nee ...
【金屬鍵】
... 15. Most plants are green in colour. Which of the following is the best explanation for this? A. Green light is reflected as it is the least effective for photosynthesis. B. Chloroplasts are green in colour. C. Plants are green in order to absorb more green light for photosynthesis. D. Chlorophyll d ...
... 15. Most plants are green in colour. Which of the following is the best explanation for this? A. Green light is reflected as it is the least effective for photosynthesis. B. Chloroplasts are green in colour. C. Plants are green in order to absorb more green light for photosynthesis. D. Chlorophyll d ...
3.091 Summary Lecture Notes, Fall 2009
... Orbital angular momentum is quantized, hence only certain orbits possible e- in stable orbits do not radiate e- change orbits by radiating or by absorbing radiation ...
... Orbital angular momentum is quantized, hence only certain orbits possible e- in stable orbits do not radiate e- change orbits by radiating or by absorbing radiation ...
Nobel Prizes 1907 Eduard Buchner, cell
... availability; allosteric effectors and/or enzyme,eg: Acetyl-CoA carboxylase (ACC), Carnitine acyltransferase I (inhibited by malonyl-CoA)); long-term regulation:(regulation of the rate of enzyme synthesis and turn-over)e.g. regulated by hormones (insulin, glucagon)|Diseases:carnitine deficiency: ina ...
... availability; allosteric effectors and/or enzyme,eg: Acetyl-CoA carboxylase (ACC), Carnitine acyltransferase I (inhibited by malonyl-CoA)); long-term regulation:(regulation of the rate of enzyme synthesis and turn-over)e.g. regulated by hormones (insulin, glucagon)|Diseases:carnitine deficiency: ina ...
Tricarboxylic acid cycle
... 1. Citrate synthase: inhibited by ATP, NADH, acyl CoA and succinyl CoA 2. Isocitrate dehydrogenase: Inhibited by ATP and NADH and activated by ADP 3. -KG dehydrogenase inhibited by NADH & succinyl CoA The availability of ADP: Important for proceeding the TCA cycle if not oxidation of NADH and FADH2 ...
... 1. Citrate synthase: inhibited by ATP, NADH, acyl CoA and succinyl CoA 2. Isocitrate dehydrogenase: Inhibited by ATP and NADH and activated by ADP 3. -KG dehydrogenase inhibited by NADH & succinyl CoA The availability of ADP: Important for proceeding the TCA cycle if not oxidation of NADH and FADH2 ...
Fatty acid catabolism leture2-3
... This condition is called “acidosis” which can lead to com or death. High concentration of ketone bodies in blood and urine is referred as “ketosis”. Due to high concentration of acetoacetate, which is converted to acetone, the breath and urine of theuntreated diabetic ...
... This condition is called “acidosis” which can lead to com or death. High concentration of ketone bodies in blood and urine is referred as “ketosis”. Due to high concentration of acetoacetate, which is converted to acetone, the breath and urine of theuntreated diabetic ...
Reaction of glycolysis
... •Mutase is an enzyme that catalyzes the intramolecular shift of a chemical group from one position to another within the same molecule such as phosphoryl group ...
... •Mutase is an enzyme that catalyzes the intramolecular shift of a chemical group from one position to another within the same molecule such as phosphoryl group ...
The Discovery of C4 Photosynthesis
... ribulose bisphosphate (RuBP) to form a six-carbon intermediate which immediately splits to form two C3 molecules of 3-phosphoglycerate. In the latter half of the next decade Marshall Hatch and Roger Slack with, PhD students Hilary Johnson and John Andrews, working at the Brisbane laboratory of the C ...
... ribulose bisphosphate (RuBP) to form a six-carbon intermediate which immediately splits to form two C3 molecules of 3-phosphoglycerate. In the latter half of the next decade Marshall Hatch and Roger Slack with, PhD students Hilary Johnson and John Andrews, working at the Brisbane laboratory of the C ...
PPT - Chris Anthony
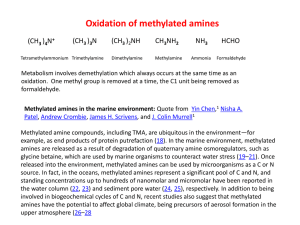
... (anaerobic Hyphomicrobia); (g) amine oxidase (in Arthrobacter and methazotrophic yeasts); (h) methylamine dehydrogenase; (i) N-methylglutamate synthase; (j) and (k) N-methylglutamate dehydrogenases. ...
... (anaerobic Hyphomicrobia); (g) amine oxidase (in Arthrobacter and methazotrophic yeasts); (h) methylamine dehydrogenase; (i) N-methylglutamate synthase; (j) and (k) N-methylglutamate dehydrogenases. ...
REVISION FOR ENERGY
... Electron Transport Chain. The Hydrogen atoms (from Krebs Cycle) combine with the coenzymes NAD and FAD to form NADH and FADH. These are then carried down the Electron Transport Chain where hydrogen is split into H+ and e‾. This takes place in the cristae of the mitochondria where three important eve ...
... Electron Transport Chain. The Hydrogen atoms (from Krebs Cycle) combine with the coenzymes NAD and FAD to form NADH and FADH. These are then carried down the Electron Transport Chain where hydrogen is split into H+ and e‾. This takes place in the cristae of the mitochondria where three important eve ...
ENERGY
... Electron Transport Chain. The Hydrogen atoms (from Krebs Cycle) combine with the coenzymes NAD and FAD to form NADH and FADH. These are then carried down the Electron Transport Chain where hydrogen is split into H+ and e‾. This takes place in the cristae of the mitochondria where three important eve ...
... Electron Transport Chain. The Hydrogen atoms (from Krebs Cycle) combine with the coenzymes NAD and FAD to form NADH and FADH. These are then carried down the Electron Transport Chain where hydrogen is split into H+ and e‾. This takes place in the cristae of the mitochondria where three important eve ...
Test 1 Study Guide Chapter 1 – Introduction
... i. Occurs on a membrane. ii. Converts energy carried by NADH and FADH2 to ATP (3/NAD, 2/FAD) (Fig. ...
... i. Occurs on a membrane. ii. Converts energy carried by NADH and FADH2 to ATP (3/NAD, 2/FAD) (Fig. ...
Test 1 Study Guide
... i. Occurs on a membrane. ii. Converts energy carried by NADH and FADH2 to ATP (3/NAD, 2/FAD) (Fig. ...
... i. Occurs on a membrane. ii. Converts energy carried by NADH and FADH2 to ATP (3/NAD, 2/FAD) (Fig. ...
CHEMISTRY OF FOOD FERMENTATION
... glycolysis. Glycolysis reduces (i.e. transfers electrons to) nicotinamide adenine dinucleotide (NAD+), forming NADH. However there is a limited supply of NAD+ available in any given cell. For glycolysis to continue, NADH must be oxidized (i.e. have electrons taken away) to regenerate the NAD+ that i ...
... glycolysis. Glycolysis reduces (i.e. transfers electrons to) nicotinamide adenine dinucleotide (NAD+), forming NADH. However there is a limited supply of NAD+ available in any given cell. For glycolysis to continue, NADH must be oxidized (i.e. have electrons taken away) to regenerate the NAD+ that i ...
Two-Metal-Ion Catalysis in Adenylyl Cyclase
... cellular functions. Recent structural studies have revealed much about the structure and function of mammalian AC but have not fully defined its active site or catalytic mechanism. Four crystal structures were determined of the catalytic domains of AC in complex with two different ATP analogs and va ...
... cellular functions. Recent structural studies have revealed much about the structure and function of mammalian AC but have not fully defined its active site or catalytic mechanism. Four crystal structures were determined of the catalytic domains of AC in complex with two different ATP analogs and va ...
How Cells Obtain Energy
... If energy is released during a chemical reaction, then the change in free energy, signified as ∆G (delta G) will be a negative number. A negative change in free energy also means that the products of the reaction have less free energy than the reactants, because they release some free energy during ...
... If energy is released during a chemical reaction, then the change in free energy, signified as ∆G (delta G) will be a negative number. A negative change in free energy also means that the products of the reaction have less free energy than the reactants, because they release some free energy during ...
DFT Chemical Reactivity Analysis of Biological Molecules in the
... enzymes; this by forming complexes with the sulfur of the thiol group of cysteine [16,17]. It is also been reported that silver can be involved in catalytic oxidation reactions resulting from the formation of disulfide bonds (R-S-S-R). It catalyzes the reaction between oxygen molecules in the thiol ...
... enzymes; this by forming complexes with the sulfur of the thiol group of cysteine [16,17]. It is also been reported that silver can be involved in catalytic oxidation reactions resulting from the formation of disulfide bonds (R-S-S-R). It catalyzes the reaction between oxygen molecules in the thiol ...
An Introduction to Metabolism
... Glucose is broken down in a series of exergonic reactions that powers the work of cells ...
... Glucose is broken down in a series of exergonic reactions that powers the work of cells ...
Cellular Pathways that Harvest Chemical Energy
... is captured in usable forms. Glycolysis does not use O2. Cellular respiration uses O2 from the environment and completely converts each pyruvate molecule to three molecules of CO2 through a set of metabolic pathways. In the process, a great deal of the energy stored in the ...
... is captured in usable forms. Glycolysis does not use O2. Cellular respiration uses O2 from the environment and completely converts each pyruvate molecule to three molecules of CO2 through a set of metabolic pathways. In the process, a great deal of the energy stored in the ...
Key area 2 * Cellular respiration
... • This is an enzyme controlled process. • E.g during glycolysis ATP is broken down to ADP + Pi and the phosphate group is used to phosphorylate the substrate of glycolysis. CFE Higher Biology ...
... • This is an enzyme controlled process. • E.g during glycolysis ATP is broken down to ADP + Pi and the phosphate group is used to phosphorylate the substrate of glycolysis. CFE Higher Biology ...
Evolution of Metabolisms - Theoretical and Computational
... Closely related organisms use similar pathways: each pair of Neisseria NG, NM, Mycobacteria (ML, MT), and Mycoplasmae (MG, MP) has the same set of pathways. Exceptions are Streptococcae (PN, ST) that are involved in ongoing sequencing projects. Thus, accessible genome data about both organisms are s ...
... Closely related organisms use similar pathways: each pair of Neisseria NG, NM, Mycobacteria (ML, MT), and Mycoplasmae (MG, MP) has the same set of pathways. Exceptions are Streptococcae (PN, ST) that are involved in ongoing sequencing projects. Thus, accessible genome data about both organisms are s ...
05 Fermentations 2008
... • when supplied with porphyrins → they form cytochromes !?! (indicating that they were originally aerobic organisms that have lost the capacity of respiration, metabolic cripples) ...
... • when supplied with porphyrins → they form cytochromes !?! (indicating that they were originally aerobic organisms that have lost the capacity of respiration, metabolic cripples) ...