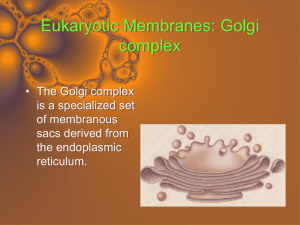
Ch. 6 Cell Respiration.notebook
... transport chain and chemiosmosis chemiosmosis: the use of a ATP Synthase (enzyme that uses the concentration gradient of H+ to create ATP) http://www.science.smith.edu/departments/Biology/Bio231/etc.html ...
... transport chain and chemiosmosis chemiosmosis: the use of a ATP Synthase (enzyme that uses the concentration gradient of H+ to create ATP) http://www.science.smith.edu/departments/Biology/Bio231/etc.html ...
Hypoxia Oxidative phosphorylation contribution to ATP production
... Portner et al. 2000. J. Exp. Biol. ...
... Portner et al. 2000. J. Exp. Biol. ...
Cellular Respiration
... • The rate of catabolism is also regulated, typically by the level of ATP in the cell –If ATP levels drop, catabolism speeds up to produce more ATP –High levels of ATP inhibit the enzyme phosphofructokinase (3rd step in glycol) –It is stimulated by high levels of AMP –Therefore, rate of glycolysis ...
... • The rate of catabolism is also regulated, typically by the level of ATP in the cell –If ATP levels drop, catabolism speeds up to produce more ATP –High levels of ATP inhibit the enzyme phosphofructokinase (3rd step in glycol) –It is stimulated by high levels of AMP –Therefore, rate of glycolysis ...
Photosynthesis
... indicating an expansion of C4 plants in the late Miocene. Isotope values are shown for carbonates (black circles) extracted from fossil soil from Pakistan, for fossil mammalian tooth enamel from Pakistan (light green squares), and for fossil horse tooth enamel from North America (green circles). The ...
... indicating an expansion of C4 plants in the late Miocene. Isotope values are shown for carbonates (black circles) extracted from fossil soil from Pakistan, for fossil mammalian tooth enamel from Pakistan (light green squares), and for fossil horse tooth enamel from North America (green circles). The ...
Cellular Respiration
... • The rate of catabolism is also regulated, typically by the level of ATP in the cell –If ATP levels drop, catabolism speeds up to produce more ATP –High levels of ATP inhibit the enzyme phosphofructokinase (3rd step in glycol) –It is stimulated by high levels of AMP –Therefore, rate of glycolysis ...
... • The rate of catabolism is also regulated, typically by the level of ATP in the cell –If ATP levels drop, catabolism speeds up to produce more ATP –High levels of ATP inhibit the enzyme phosphofructokinase (3rd step in glycol) –It is stimulated by high levels of AMP –Therefore, rate of glycolysis ...
Electron transport chain…
... Chemiosmotic hypothesis • is the most widely accepted hypothesis to explain oxidative phosphorylation – electron transport chain organized so protons move outward from the mitochondrial matrix as electrons are transported down the chain – proton expulsion during electron transport results in the for ...
... Chemiosmotic hypothesis • is the most widely accepted hypothesis to explain oxidative phosphorylation – electron transport chain organized so protons move outward from the mitochondrial matrix as electrons are transported down the chain – proton expulsion during electron transport results in the for ...
Sample lab - eScience Labs
... Cellular Respiration 8. Use the permanent marker to label the fourth 250 mL beaker as Yeast. 9. Fill this beaker with 175 mL of warm tap water. The exact temperature does not matter, but the water should be warm to the touch. 10. Open the yeast package, and use the measuring spoon to measure and po ...
... Cellular Respiration 8. Use the permanent marker to label the fourth 250 mL beaker as Yeast. 9. Fill this beaker with 175 mL of warm tap water. The exact temperature does not matter, but the water should be warm to the touch. 10. Open the yeast package, and use the measuring spoon to measure and po ...
Metabolism & Enzymes - San Juan Unified School District
... (c) A multi-step open hydroelectric system. Cellular respiration is analogous to this system: Glucose is broken down in a series of exergonic reactions that power the work of the cell. The product of each reaction becomes the reactant for the next, so no reaction reaches equilibrium. ...
... (c) A multi-step open hydroelectric system. Cellular respiration is analogous to this system: Glucose is broken down in a series of exergonic reactions that power the work of the cell. The product of each reaction becomes the reactant for the next, so no reaction reaches equilibrium. ...
Interpretation of electron density with stereographic roadmap
... residues of CVA21 are plotted onto a stereographic projection and colored from blue (135 Å) to red (165 Å) based on their maximum radial distance from the center of the virus. (B) The location of the “pocket factor” in a 3.2 Å resolution electron density map of CVA21 crystals (Xiao et al., 2005a) sh ...
... residues of CVA21 are plotted onto a stereographic projection and colored from blue (135 Å) to red (165 Å) based on their maximum radial distance from the center of the virus. (B) The location of the “pocket factor” in a 3.2 Å resolution electron density map of CVA21 crystals (Xiao et al., 2005a) sh ...
basic biochemistry - Personal Webspace for QMUL
... Glycolysis converts glucose to pyruvate and creates ATP Occurs in the cytosol Involves six-carbon and three-carbon molecules All intermediates molecules are phosphorylated At a critical SECOND STAGE in the process TWO three-carbon molecules are produced Both can get used in the next stag ...
... Glycolysis converts glucose to pyruvate and creates ATP Occurs in the cytosol Involves six-carbon and three-carbon molecules All intermediates molecules are phosphorylated At a critical SECOND STAGE in the process TWO three-carbon molecules are produced Both can get used in the next stag ...
ATPase - cloudfront.net
... http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/P/Proteins.html ...
... http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/P/Proteins.html ...
Lecture 6
... occurs. The 2 moles of NADH produced by glyceraldehyde-3-phosphate dehydrogenase are oxidized in the electron transport chain back to NAD +. The electron transport chain generates a proton gradient that drives the synthesis of 5 ATP molecules from ADP and Pi. Furthermore, the pyruvate formed by glyc ...
... occurs. The 2 moles of NADH produced by glyceraldehyde-3-phosphate dehydrogenase are oxidized in the electron transport chain back to NAD +. The electron transport chain generates a proton gradient that drives the synthesis of 5 ATP molecules from ADP and Pi. Furthermore, the pyruvate formed by glyc ...
Fatty Acid Catabolism
... Chylomicrons contain phospholipids and proteins on the surface so that the hydrophilic surfaces are in contact with water. The hydrophobic molecules are enclosed in the interior. The lone hydroxyl group of cholesterol molecules is oriented towards the outer surface shown here as black dots. Chylomic ...
... Chylomicrons contain phospholipids and proteins on the surface so that the hydrophilic surfaces are in contact with water. The hydrophobic molecules are enclosed in the interior. The lone hydroxyl group of cholesterol molecules is oriented towards the outer surface shown here as black dots. Chylomic ...
03-232 Exam III 2013 Name:__________________________
... iii) What is the next step in the energy flow? (1pt, no need to give details, just state how the energy is stored, you can provide the details in Q13) NADH/FADH2 enter the electron transport chain. When these are oxidized back to NAD+/FAD, the energy released is stored in a H+ gradient across the in ...
... iii) What is the next step in the energy flow? (1pt, no need to give details, just state how the energy is stored, you can provide the details in Q13) NADH/FADH2 enter the electron transport chain. When these are oxidized back to NAD+/FAD, the energy released is stored in a H+ gradient across the in ...
Preview Sample 1 - Test Bank, Manual Solution, Solution Manual
... Ans: An ion is an atom that has either gained one or more electrons or given up one or more electrons. A molecule results from two or more atoms sharing electrons. 10. What is a “cation”? A) Any atom that has lost or gained electrons, resulting in a negatively-charged ion or a positively-charged ion ...
... Ans: An ion is an atom that has either gained one or more electrons or given up one or more electrons. A molecule results from two or more atoms sharing electrons. 10. What is a “cation”? A) Any atom that has lost or gained electrons, resulting in a negatively-charged ion or a positively-charged ion ...
Redox (short for reduction–oxidation reaction) is a
... to extract the metal. In other words, ore was "reduced" to metal. Antoine Lavoisier (1743–1794) showed that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of reduction then became generalized to ...
... to extract the metal. In other words, ore was "reduced" to metal. Antoine Lavoisier (1743–1794) showed that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of reduction then became generalized to ...
Lecture 1 - Алтайский государственный технический
... The diameters of atomic nuclei are about 10-4A. Thus, the nuclei are about 0.01% the diameter of the atom as a whole. If the nucleus had a diameter equal to that of a pinhead, then the atom itself would have a diameter of some 10 meters (about 39 and a half feet). The nucleus of an atom is therefor ...
... The diameters of atomic nuclei are about 10-4A. Thus, the nuclei are about 0.01% the diameter of the atom as a whole. If the nucleus had a diameter equal to that of a pinhead, then the atom itself would have a diameter of some 10 meters (about 39 and a half feet). The nucleus of an atom is therefor ...
Chapter 8
... form: OH group of the anomeric C is on OPPOSITE side of ring from CH2OH form: OH group of the anomeric C is on SAME side of ring from CH2OH ...
... form: OH group of the anomeric C is on OPPOSITE side of ring from CH2OH form: OH group of the anomeric C is on SAME side of ring from CH2OH ...
GOALS FOR LECTURE 7:
... A variety of specific enzymes on the surface of the intestinal cells hydrolyze various disaccharides. Lactose in milk is a major source of nutrition for young mammals including humans, and it is split by β-galactosidase (also called lactase) in the intestine into galactose and glucose. Most adult ma ...
... A variety of specific enzymes on the surface of the intestinal cells hydrolyze various disaccharides. Lactose in milk is a major source of nutrition for young mammals including humans, and it is split by β-galactosidase (also called lactase) in the intestine into galactose and glucose. Most adult ma ...
Carbohydrate Metabolism Glucose Metabolism Oxidation of Glucose
... 1. Hexokinase has high affinity for glucose ( Km ≈ 0.04 mM ) . Since the resting level for blood glucose is about 5mM , therefore hexokinase would be expected to be fully active for all body cells at the resting level and the liver would not be competing with other cells for glucose . On the other h ...
... 1. Hexokinase has high affinity for glucose ( Km ≈ 0.04 mM ) . Since the resting level for blood glucose is about 5mM , therefore hexokinase would be expected to be fully active for all body cells at the resting level and the liver would not be competing with other cells for glucose . On the other h ...
Lec 1-10 Problem Set Answers
... e) When the 500 turns is complete, how many OAA molecules are present? f) You over hear a student going “I don't get it, we put 500 acetyl groups into that single Krebs cycle, and at the end of it, why don’t we have 500 OAAs? What kind of lame metabolic pathway is this anyway?” Explain 7) The glyoxa ...
... e) When the 500 turns is complete, how many OAA molecules are present? f) You over hear a student going “I don't get it, we put 500 acetyl groups into that single Krebs cycle, and at the end of it, why don’t we have 500 OAAs? What kind of lame metabolic pathway is this anyway?” Explain 7) The glyoxa ...