Lec 1-10 Problem Set Answers
... e) When the 500 turns is complete, how many OAA molecules are present? f) You over hear a student going “I don't get it, we put 500 acetyl groups into that single Krebs cycle, and at the end of it, why don’t we have 500 OAAs? What kind of lame metabolic pathway is this anyway?” Explain 7) The glyoxa ...
... e) When the 500 turns is complete, how many OAA molecules are present? f) You over hear a student going “I don't get it, we put 500 acetyl groups into that single Krebs cycle, and at the end of it, why don’t we have 500 OAAs? What kind of lame metabolic pathway is this anyway?” Explain 7) The glyoxa ...
Ch. 25
... carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor ...
... carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor ...
Krebs Cycle
... carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor ...
... carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor ...
ENERGY-PRODUCING ABILITY OF BACTERIA
... used as a common means of producing ATP through photophosphorylation. Once the amount of NADPH exceeds the level of NADP+, normal ATP production is often slow down or halted. NADP+ must be available as a reducing cofactor for the reaction and its decrease leads to a concomitant reduction of photopho ...
... used as a common means of producing ATP through photophosphorylation. Once the amount of NADPH exceeds the level of NADP+, normal ATP production is often slow down or halted. NADP+ must be available as a reducing cofactor for the reaction and its decrease leads to a concomitant reduction of photopho ...
The role of ATP in metabolism
... amides, glycosides or esters, (eg, see Eqn 13). Direct condensation reactions (which do not involve ATP) have equilibrium constants which are usually very much less than unity, reflecting the fact that water must be liberated into a medium in which its chemical potential is already very high. It is ...
... amides, glycosides or esters, (eg, see Eqn 13). Direct condensation reactions (which do not involve ATP) have equilibrium constants which are usually very much less than unity, reflecting the fact that water must be liberated into a medium in which its chemical potential is already very high. It is ...
Export To Word
... This Khan Academy video describes how the pyruvate produced in glycolysis undergoes oxidation to produce Acetyl CoA. The video then explains what occurs when Acetyl CoA enters the Kreb's cycle and how NADH and FADH2 are produced. This Khan Academy video explains how the NADH And FADH2 that were made ...
... This Khan Academy video describes how the pyruvate produced in glycolysis undergoes oxidation to produce Acetyl CoA. The video then explains what occurs when Acetyl CoA enters the Kreb's cycle and how NADH and FADH2 are produced. This Khan Academy video explains how the NADH And FADH2 that were made ...
ATP
... NAD (nicotinamide adenine dinucleotide), FAD (Flavine adenine dinucleotide, coenzyme Q and iron-containing proteins (cytochromes) Oxidative phosphorylation: generation of ATP by H atoms in an electron transport system in which H atoms link with O atoms to form water. Oxygen is necessary as the final ...
... NAD (nicotinamide adenine dinucleotide), FAD (Flavine adenine dinucleotide, coenzyme Q and iron-containing proteins (cytochromes) Oxidative phosphorylation: generation of ATP by H atoms in an electron transport system in which H atoms link with O atoms to form water. Oxygen is necessary as the final ...
Lecture 27
... Mammals have a second control at OMP decarboxylase (competitively inhibited by UMP and CMP) PRPP also affects rate of OMP production, so, ADP and GDP will inhibit PRPP production. ...
... Mammals have a second control at OMP decarboxylase (competitively inhibited by UMP and CMP) PRPP also affects rate of OMP production, so, ADP and GDP will inhibit PRPP production. ...
rll 24.5 The citric ocid cycle
... electron transport chain can opelate. \Mhen there is no oxygen available to drain electrons from NADH and FADH2 in respiration, the electron carriers of the electron transport chain become completely reduced. More electrons cannot be passed down the chain, and oxidative phosphorylation stops. Howeve ...
... electron transport chain can opelate. \Mhen there is no oxygen available to drain electrons from NADH and FADH2 in respiration, the electron carriers of the electron transport chain become completely reduced. More electrons cannot be passed down the chain, and oxidative phosphorylation stops. Howeve ...
medbiochem exam 1, 2000
... 47. Which of the following statements is TRUE? Enzyme catalysis of a chemical reaction: A. increases the energy of the transition state. B. decreases the change in free energy of the reaction. C. increases the change in free energy of the reaction. D. decreases the change in the free energy of activ ...
... 47. Which of the following statements is TRUE? Enzyme catalysis of a chemical reaction: A. increases the energy of the transition state. B. decreases the change in free energy of the reaction. C. increases the change in free energy of the reaction. D. decreases the change in the free energy of activ ...
Cellular Respiration
... a. What provided the spark to start the candle burning? b. What provides the fuel for the burning candle? 2. Is the burning candle giving off any type of energy? If so, what kind(s) of energy are being released? 3. Place the beaker or flask over the candle. What happens? 4. What caused the candle to ...
... a. What provided the spark to start the candle burning? b. What provides the fuel for the burning candle? 2. Is the burning candle giving off any type of energy? If so, what kind(s) of energy are being released? 3. Place the beaker or flask over the candle. What happens? 4. What caused the candle to ...
3. DarkReaction
... movement of K+ ions into the cells Generally stomata are open during the day and closed at night ...
... movement of K+ ions into the cells Generally stomata are open during the day and closed at night ...
Chapters11-Glycolysis-2014
... The citric acid cycle, aka the tricarboxylic acid cycle (TCA), or the Krebs cycle: Series of chemical reactions used by all aerobic organisms to generate energy. It works by the oxidation of acetate derived from carbohydrates, fats and proteins into CO2 and G in the form of ATP. The cycle also provi ...
... The citric acid cycle, aka the tricarboxylic acid cycle (TCA), or the Krebs cycle: Series of chemical reactions used by all aerobic organisms to generate energy. It works by the oxidation of acetate derived from carbohydrates, fats and proteins into CO2 and G in the form of ATP. The cycle also provi ...
... Choice A: The energy released by degradative pathways is directly captured on which types of compounds? Briefly explain how the energy on these compounds is converted to a hydrogen ion (proton) gradient across a membrane during electron transport. Choice B: Briefly explain how the hydrogen ion gradi ...
Cellular Respiration G! Cellular Respiration
... Energy from the mitochondrion is also stored in the form of ATP. Thirty ATP molecules are produced for every two molecules of pyruvic acid and remember that the two pyruvic molecules come from one molecule of glucose. 6. Analyze: Cellular respiration involves two phases. Anaerobic respiration does n ...
... Energy from the mitochondrion is also stored in the form of ATP. Thirty ATP molecules are produced for every two molecules of pyruvic acid and remember that the two pyruvic molecules come from one molecule of glucose. 6. Analyze: Cellular respiration involves two phases. Anaerobic respiration does n ...
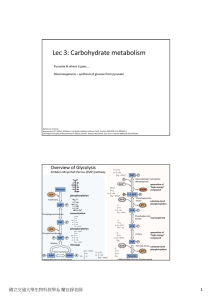
Lec 3: Carbohydrate metabolism
... intermediates of TCA cycle). Anaplerotic reactions are important as intermediates of TCA cycle are used to ...
... intermediates of TCA cycle). Anaplerotic reactions are important as intermediates of TCA cycle are used to ...
Respiration and Lipid Metabolism - Roberto Cezar | Fisiologista
... free energy, much of which is conserved through the synthesis of ATP from ADP and Pi (inorganic phosphate) catalyzed by the enzyme ATP synthase. Collectively the redox reactions of the electron transport chain and the synthesis of ATP are called oxidative phosphorylation. This final stage completes ...
... free energy, much of which is conserved through the synthesis of ATP from ADP and Pi (inorganic phosphate) catalyzed by the enzyme ATP synthase. Collectively the redox reactions of the electron transport chain and the synthesis of ATP are called oxidative phosphorylation. This final stage completes ...
Chapter 25
... carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor ...
... carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor ...
Lecture 33 Carbohydrates1
... 1. When NADPH is needed, ribulose-5P is converted back into glucose6P to maintain flux through the pathway. 2. If ATP and NADPH are needed (which would be the case for most anabolic pathways), then some of the ribulose-5P is used to synthesis hexose phosphates for glycolysis. 3. If the cell needs to ...
... 1. When NADPH is needed, ribulose-5P is converted back into glucose6P to maintain flux through the pathway. 2. If ATP and NADPH are needed (which would be the case for most anabolic pathways), then some of the ribulose-5P is used to synthesis hexose phosphates for glycolysis. 3. If the cell needs to ...
enzyme substrate
... Most of the ATP made by the cell is used in the production of new cellular components Amphibolic pathways bridge the reactions that lead to the breakdown and synthesis of carbohydrates, lipids, proteins and nucleus. These pathways allow for the simultaneous reactions to occur in which the breakdown ...
... Most of the ATP made by the cell is used in the production of new cellular components Amphibolic pathways bridge the reactions that lead to the breakdown and synthesis of carbohydrates, lipids, proteins and nucleus. These pathways allow for the simultaneous reactions to occur in which the breakdown ...
Cellular respiration
... Chlorophyll and other pigments within the thylakoid membranes absorb solar energy. Conversion of CO2 to carbohydrates occurs in the stroma. Chloroplast outer membrane inner membrane ...
... Chlorophyll and other pigments within the thylakoid membranes absorb solar energy. Conversion of CO2 to carbohydrates occurs in the stroma. Chloroplast outer membrane inner membrane ...
Document
... - They accept an electron and reduce to (Fe2+). - They pass the electron to the next cytochrome and they are oxidized back to Fe3+. ...
... - They accept an electron and reduce to (Fe2+). - They pass the electron to the next cytochrome and they are oxidized back to Fe3+. ...
Chemistry Notes for the Whole Year Powerpoint
... electrons so as to have eight electrons in their outer electron shell. This means that all atoms, in a Lewis structure, must have eight valence electrons around them (they can be either bonded or lone pair electrons). • Hydrogen and helium are exceptions to the octet rule. There is one more element ...
... electrons so as to have eight electrons in their outer electron shell. This means that all atoms, in a Lewis structure, must have eight valence electrons around them (they can be either bonded or lone pair electrons). • Hydrogen and helium are exceptions to the octet rule. There is one more element ...