File
... become charged by induction because charged particles are not free to move within an insulator. However, if an insulator is in the midst of an electric field, the individual molecules, while not able to move freely, may orient themselves so that there is a polarization of charge. ...
... become charged by induction because charged particles are not free to move within an insulator. However, if an insulator is in the midst of an electric field, the individual molecules, while not able to move freely, may orient themselves so that there is a polarization of charge. ...
Thermal Analysis Infrared Microscopy During device functioning, the
... It is important to note that all these special means (and others) must be used only to identify the failure mechanism. In other words, the special means must be needed by the logic of the failure analysis. A tendency to embellish the failure reports by adding “beautiful” results (impressive pictures ...
... It is important to note that all these special means (and others) must be used only to identify the failure mechanism. In other words, the special means must be needed by the logic of the failure analysis. A tendency to embellish the failure reports by adding “beautiful” results (impressive pictures ...
Basics of Semiconductors_1
... But after doping one clearly dominates the other making it negligible ...
... But after doping one clearly dominates the other making it negligible ...
Atom The smallest part of an element that can exist on its own
... Highest significant peak where the molecule has lost 1 electron but has not broken up Base peak Highest peak in the mass spectrum - If the sample is an element each line represents an isotope of the element - Molecules broken up into fragments make fragmentation patterns on the mass spectrum(used to ...
... Highest significant peak where the molecule has lost 1 electron but has not broken up Base peak Highest peak in the mass spectrum - If the sample is an element each line represents an isotope of the element - Molecules broken up into fragments make fragmentation patterns on the mass spectrum(used to ...
Chemistry for BIOS 302
... The next two shells hold up to 8 electrons. The first three shells thus hold 2 + 8 + 8 = 18 electrons, which corresponds to argon. – Things get more complex above this point, but nearly all the biologically important elements use only the first 3 electron shells. – Big exception: metals such as iron ...
... The next two shells hold up to 8 electrons. The first three shells thus hold 2 + 8 + 8 = 18 electrons, which corresponds to argon. – Things get more complex above this point, but nearly all the biologically important elements use only the first 3 electron shells. – Big exception: metals such as iron ...
Mass Spectrum – Interpretation
... Fluorine has only one isotope (19F), so the mass spectrum of F2 will show only two peaks one at 19 and the other at 38 (which one is bigger cannot be predicted – depends on how stable the F2 + ion is - the diagram below is just a sketch). ...
... Fluorine has only one isotope (19F), so the mass spectrum of F2 will show only two peaks one at 19 and the other at 38 (which one is bigger cannot be predicted – depends on how stable the F2 + ion is - the diagram below is just a sketch). ...
200 Ways to Pass the Chemistry
... sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl It loses its 1 valence electron l ...
... sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl It loses its 1 valence electron l ...
The only sure evidence that a chemical reaction has occured is
... What is shown by A in Graph 1? What is shown by B in Graph 1? What type of reaction is shown in Graph 1? Which graph illustrates the type of reaction that occurs when wood burns? ...
... What is shown by A in Graph 1? What is shown by B in Graph 1? What type of reaction is shown in Graph 1? Which graph illustrates the type of reaction that occurs when wood burns? ...
Answers
... Q3. Across a field, as more electrons occupy the same principal energy level, they are accompanied by additional protons in the nuclei. All outer electrons experience greater attraction to the nucleus (effective nuclear charge is increasing), and the radius decreases. After the noble gas in each row ...
... Q3. Across a field, as more electrons occupy the same principal energy level, they are accompanied by additional protons in the nuclei. All outer electrons experience greater attraction to the nucleus (effective nuclear charge is increasing), and the radius decreases. After the noble gas in each row ...
Unit Two Objectives
... 1. The Periodic Table: What is the Periodic Law? The Periodic Law states that when the elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties. a. The horizontal rows are called the periods. There are seven periods. Going a ...
... 1. The Periodic Table: What is the Periodic Law? The Periodic Law states that when the elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties. a. The horizontal rows are called the periods. There are seven periods. Going a ...
SOL Essential Knowledge
... 2. Ionization energy is the energy required to remove the most easily held electron. 3. Elements with low ionization energy form ions easily. F. Recognize that transition metals can have multiple oxidation states. G. Summarize the following concepts about covalent bonding: 1. Covalent bonds involve ...
... 2. Ionization energy is the energy required to remove the most easily held electron. 3. Elements with low ionization energy form ions easily. F. Recognize that transition metals can have multiple oxidation states. G. Summarize the following concepts about covalent bonding: 1. Covalent bonds involve ...
Electricity - Cobb Learning
... electricity as electric charges transfer from one object to another This means that when a negatively charged object and a positively charged object are brought together, electrons transfer until both objects have the same charge ...
... electricity as electric charges transfer from one object to another This means that when a negatively charged object and a positively charged object are brought together, electrons transfer until both objects have the same charge ...
Final Exam Review Day 1
... Mg+2 Valence electrons are electrons in the outer shell. Determine the number of valence electrons and draw the dot diagrams for the ...
... Mg+2 Valence electrons are electrons in the outer shell. Determine the number of valence electrons and draw the dot diagrams for the ...
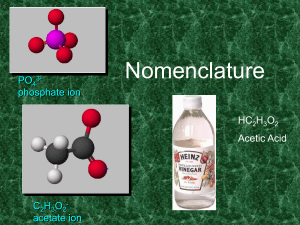
Naming Chemical Compounds
... Write the formula for manganese (III) dihydrogen phosphite All formulae in this course consists of a positive particle (cation) and a negative particle (anion). The positive particle is Mn3+. This is determined from the Roman Numeral in the name. 3- 1- H PO 1- H PO 1Mn3+ H PO PO ...
... Write the formula for manganese (III) dihydrogen phosphite All formulae in this course consists of a positive particle (cation) and a negative particle (anion). The positive particle is Mn3+. This is determined from the Roman Numeral in the name. 3- 1- H PO 1- H PO 1Mn3+ H PO PO ...
HIGHER TIER CHEMISTRY MINI-MOCK UNIT 2
... The graph below shows the results of four experiments, 1 to 4. In each experiment the amount of calcium carbonate, the volume of acid and the concentration of the acid were kept the same but the temperature of the acid was changed each time. The calcium carbonate was in the form of small lumps of ma ...
... The graph below shows the results of four experiments, 1 to 4. In each experiment the amount of calcium carbonate, the volume of acid and the concentration of the acid were kept the same but the temperature of the acid was changed each time. The calcium carbonate was in the form of small lumps of ma ...
ionization 12.3.1
... Describes the process whereby new ionized species are formed when gaseous molecules interact with ions. The process may involve transfer of an electron, a proton or other charged species between the reactants. When a positive ion results from chemical ionization the term may be used without qualific ...
... Describes the process whereby new ionized species are formed when gaseous molecules interact with ions. The process may involve transfer of an electron, a proton or other charged species between the reactants. When a positive ion results from chemical ionization the term may be used without qualific ...
Chem 150 - Fall 2015 Exam I
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
8B31A38F-1279-3B00-CDA90244BEA11A7B
... • There are 3 forms bonding atoms: • Ionic—complete transfer of 1 or more electrons from one atom to another (one loses, the other gains) • Covalent—some valence electrons shared between atoms • Metallic – holds atoms of a metal together ...
... • There are 3 forms bonding atoms: • Ionic—complete transfer of 1 or more electrons from one atom to another (one loses, the other gains) • Covalent—some valence electrons shared between atoms • Metallic – holds atoms of a metal together ...
Part II - Web site of Dr. Charles Berks
... Binary ionic bonding forms between a metal and a nonmetal whose electronegativities widely differ. This type of bonding produces clusters or aggregates of ions that are more stable than the isolated atoms from which they are formed. This stability is due to the electrostatic attractions that exist b ...
... Binary ionic bonding forms between a metal and a nonmetal whose electronegativities widely differ. This type of bonding produces clusters or aggregates of ions that are more stable than the isolated atoms from which they are formed. This stability is due to the electrostatic attractions that exist b ...
specimen
... ‘Dot-and-cross’ diagrams are used to model which electrons are present in the ion. Draw a ‘dot-and-cross’ diagram, including outer electron shells only, to show the ions present in magnesium chloride, MgCl2. ...
... ‘Dot-and-cross’ diagrams are used to model which electrons are present in the ion. Draw a ‘dot-and-cross’ diagram, including outer electron shells only, to show the ions present in magnesium chloride, MgCl2. ...
Review Packet
... 35. Element X has two natural isotopes. The isotope with mass 62.9265 amu has a relative abundance of 69.17%. The isotope with mass 64.9278 amu has a relative abundance of 30.83%. What is the average atomic mass of element X? ...
... 35. Element X has two natural isotopes. The isotope with mass 62.9265 amu has a relative abundance of 69.17%. The isotope with mass 64.9278 amu has a relative abundance of 30.83%. What is the average atomic mass of element X? ...
Electric Charge
... touch a door knob, we often experience a spark • This process is called charging ...
... touch a door knob, we often experience a spark • This process is called charging ...
Chapter 3
... According to principle, electrons occupy the orbitals of lowest energy first. It dictates that for every further proton in the nucleus, there is an electron in an orbital of that atom. This principle also dictates the chemical and physical properties of an element, and its position in the periodic t ...
... According to principle, electrons occupy the orbitals of lowest energy first. It dictates that for every further proton in the nucleus, there is an electron in an orbital of that atom. This principle also dictates the chemical and physical properties of an element, and its position in the periodic t ...