Chapter 4 Nomenclature and Chemical Equations
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
CHEM1405 2005-J-3 June 2005 • Ammonia (NH 3) has a boiling
... much larger atom that H or C and hence has more electrons and these are held further from the nucleus. The electron cloud in I2 is, therefore, much more polarisable leading to stronger dispersion forces in I2. and a higher melting point. NaCl has relatively strong ionic bonds between all of the Na+ ...
... much larger atom that H or C and hence has more electrons and these are held further from the nucleus. The electron cloud in I2 is, therefore, much more polarisable leading to stronger dispersion forces in I2. and a higher melting point. NaCl has relatively strong ionic bonds between all of the Na+ ...
The Egyptian American International School
... 2. Probability maps indicate the likelihood of finding the electron at a given point in space. 3. The size of an atom can be described by a surface that contains 90% of the total electron probability. 11.4 Electron Configurations and Atomic Properties Atomic energy levels are broken down into prin ...
... 2. Probability maps indicate the likelihood of finding the electron at a given point in space. 3. The size of an atom can be described by a surface that contains 90% of the total electron probability. 11.4 Electron Configurations and Atomic Properties Atomic energy levels are broken down into prin ...
2011 Lecture 22: Transport in Bulk Electrolytes
... A subtle consequence of quasi-electroneutrality is that the bulk electric field generally does not satisfy Maxwell’s equations. In particular, in a linear dielectric medium, one should properly impose Poisson’s equation, −ε∇2 φ = ρe (where ε is the permittivity) with an electrostatic boundary condit ...
... A subtle consequence of quasi-electroneutrality is that the bulk electric field generally does not satisfy Maxwell’s equations. In particular, in a linear dielectric medium, one should properly impose Poisson’s equation, −ε∇2 φ = ρe (where ε is the permittivity) with an electrostatic boundary condit ...
C4C5C6
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _____. A) protons B) electrons when neutral ...
... B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _____. A) protons B) electrons when neutral ...
Regents Chemistry
... Be able to explain why energy is absorbed when a bond is broken & energy is released when a bond is formed. o ...
... Be able to explain why energy is absorbed when a bond is broken & energy is released when a bond is formed. o ...
CHEMISTRY FALL FINAL PRACTICE 2016
... List the seven diatomic molecules and indicate the number of bonds between them. ...
... List the seven diatomic molecules and indicate the number of bonds between them. ...
Reactions In Aqueous Solution
... silicates, and sulfides are insoluble. (6) Sulfates are soluble except for calcium and barium. ...
... silicates, and sulfides are insoluble. (6) Sulfates are soluble except for calcium and barium. ...
Chemical Equations and Reactions
... greater the activity is. • Metals: the greater the activity, the greater it loses electrons (to form cations) • Non-metals: the greater the activity, the greater it gains electrons (to form anions) • Activity series: a list of which elements a particular element can replace in a single replacement r ...
... greater the activity is. • Metals: the greater the activity, the greater it loses electrons (to form cations) • Non-metals: the greater the activity, the greater it gains electrons (to form anions) • Activity series: a list of which elements a particular element can replace in a single replacement r ...
blood - SCH4U1-02-2010
... - The ideal pH level for blood is 7.35-7.45 - The higher the pH of a solution, the more electrical resistance that solution holds. - If pH level is either alkaline or acid, you will become ill, perhaps even die. 6. Electrolyte -Dietary Electrolytes and Blood Pressure. Electrolyte is a "medical/scien ...
... - The ideal pH level for blood is 7.35-7.45 - The higher the pH of a solution, the more electrical resistance that solution holds. - If pH level is either alkaline or acid, you will become ill, perhaps even die. 6. Electrolyte -Dietary Electrolytes and Blood Pressure. Electrolyte is a "medical/scien ...
chapter-2 - HCC Learning Web
... Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be appro ...
... Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be appro ...
200 Things to Know to Pass the Chemistry Regents
... Metallic bonding occurs between atoms of sulfur sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structu ...
... Metallic bonding occurs between atoms of sulfur sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structu ...
200 Ways to Pass the Chemistry
... Metallic bonding occurs between atoms of sulfur sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structu ...
... Metallic bonding occurs between atoms of sulfur sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structu ...
200things2know
... Metallic bonding occurs between atoms of sulfur sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structu ...
... Metallic bonding occurs between atoms of sulfur sodium fluoride sodium carbon 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structu ...
NCEA Level 1 Chemistry (90933) 2014
... • Correct ion configurations. (Brackets with charge are not required.) ...
... • Correct ion configurations. (Brackets with charge are not required.) ...
Zumdahl Chapter
... First Year Chemistry Podcast DVD Featuring Jonathan Bergmann and Aaron Sams from Peak Educational Consulting LLC All Rights Reserved © This is an interactive page that allows you to get to all of the content on this DVD. Click to each unit packet or podcast. The podcasts require Quicktime and the pa ...
... First Year Chemistry Podcast DVD Featuring Jonathan Bergmann and Aaron Sams from Peak Educational Consulting LLC All Rights Reserved © This is an interactive page that allows you to get to all of the content on this DVD. Click to each unit packet or podcast. The podcasts require Quicktime and the pa ...
Chemistry Review ATOMS
... • Elements have the same # of valence electrons • Elements share similar chemical properties including reactivity ...
... • Elements have the same # of valence electrons • Elements share similar chemical properties including reactivity ...
Name: Period:______ PHYSICAL SCIENCE 1st Semester Final
... Mendeleev arranged the elements into rows in order of increasing mass so that the elements with similar properties were in the same column. The close match between Mendeleev’s predictions and the actual properties of new elements showed how useful his periodic table could be. In the modern per ...
... Mendeleev arranged the elements into rows in order of increasing mass so that the elements with similar properties were in the same column. The close match between Mendeleev’s predictions and the actual properties of new elements showed how useful his periodic table could be. In the modern per ...
2.3 Atomic and Molecular Collisions
... Ion beam lifetime studies and Schottky noise diagnostics. Ion beam storage lifetimes in the CSR were observed (Fig. 3) to reach up to 45 min. Stored-beam signals could be observed up to three hours following a single injection. These times are largely sufficient for the envisaged experiments and alm ...
... Ion beam lifetime studies and Schottky noise diagnostics. Ion beam storage lifetimes in the CSR were observed (Fig. 3) to reach up to 45 min. Stored-beam signals could be observed up to three hours following a single injection. These times are largely sufficient for the envisaged experiments and alm ...
Dissociation
... — If the mixing results in a combination of ions that forms an insoluble compound, a double replacement reaction (remember those?!?) will occur and the end result will be the formation of a precipitate — Precipitation occurs because the forces of attraction between the insoluble ion pair combination ...
... — If the mixing results in a combination of ions that forms an insoluble compound, a double replacement reaction (remember those?!?) will occur and the end result will be the formation of a precipitate — Precipitation occurs because the forces of attraction between the insoluble ion pair combination ...
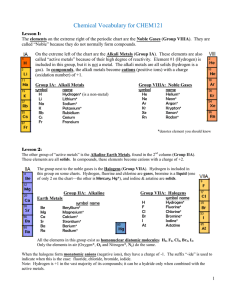
Vocabulary CHEM121
... Molecular vs. Ionic Compounds Compounds may be divided into 2 general types: 1. Molecular (covalent) compounds are combinations of non-metals 2. Ionic (contains ions) includes: Acids: anything giving H+ when dissolved in water Bases: anything giving OH- when dissolved in water Salts: all other ionic ...
... Molecular vs. Ionic Compounds Compounds may be divided into 2 general types: 1. Molecular (covalent) compounds are combinations of non-metals 2. Ionic (contains ions) includes: Acids: anything giving H+ when dissolved in water Bases: anything giving OH- when dissolved in water Salts: all other ionic ...
Lecture 9. Redox chemistry
... •Iron, a common construction metal often used in forming steel alloys, corrodes by being oxidized to ions of iron by oxygen. •This corrosion is even faster in the presence of salts and acids, because these materials make electrically conductive solutions that make electron transfer easy ...
... •Iron, a common construction metal often used in forming steel alloys, corrodes by being oxidized to ions of iron by oxygen. •This corrosion is even faster in the presence of salts and acids, because these materials make electrically conductive solutions that make electron transfer easy ...