Review Sheet: Unit 6 Name__________________ CHEMISTRY: A
... Special abbreviations are used to show the physical state of a substance in a reaction. The symbol for a liquid is ____________; for a solid, ____________; for a gas, ____________ or ____________; and for a precipitate (an ____________ solid), a ____________ or ____________. A substance that is dis ...
... Special abbreviations are used to show the physical state of a substance in a reaction. The symbol for a liquid is ____________; for a solid, ____________; for a gas, ____________ or ____________; and for a precipitate (an ____________ solid), a ____________ or ____________. A substance that is dis ...
nuc_alchemy_talk-fgs-dec07
... Nuclear Fusion creates energy up to A~56 (Z=26 = Iron) If the star is hot enough, nuclear fusion will fuel the star and create elements up to A~56 ...
... Nuclear Fusion creates energy up to A~56 (Z=26 = Iron) If the star is hot enough, nuclear fusion will fuel the star and create elements up to A~56 ...
High School Curriculum Standards: Chemistry
... 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that matter is composed of particles of four elements (earth, air, water, and fire) and that these particles are in continua ...
... 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that matter is composed of particles of four elements (earth, air, water, and fire) and that these particles are in continua ...
8.P.1.1Homework for Website
... 13. Which BEST explains how atoms combine to form compounds? A. The atoms in the compound share electrons B. The atoms in the compound share neutrons C. The atoms in the compound share protons 14. The compound propane has three carbon atoms and eight hydrogen atoms. What is the chemical formula of p ...
... 13. Which BEST explains how atoms combine to form compounds? A. The atoms in the compound share electrons B. The atoms in the compound share neutrons C. The atoms in the compound share protons 14. The compound propane has three carbon atoms and eight hydrogen atoms. What is the chemical formula of p ...
Document
... Nuclide: a nucleus with a certain atomic and mass number (a given number of protons and neutrons) Isotopes: have same atomic number, different mass #'s (same number of ______, different number of _____) ...
... Nuclide: a nucleus with a certain atomic and mass number (a given number of protons and neutrons) Isotopes: have same atomic number, different mass #'s (same number of ______, different number of _____) ...
AP Exam One Retake Qualifying Assignment
... most metals at room temperature sodium metal and chlorine gas in the formation of sodium chloride paper turns yellow after sitting in the sun for long periods of time breaking a glass bottle photosynthesis turns carbon dioxide and water into sugar and oxygen sugar and oxygen in photosynthesis mercur ...
... most metals at room temperature sodium metal and chlorine gas in the formation of sodium chloride paper turns yellow after sitting in the sun for long periods of time breaking a glass bottle photosynthesis turns carbon dioxide and water into sugar and oxygen sugar and oxygen in photosynthesis mercur ...
1 - Academics
... REVIEW PROBLEMS FOR FINAL: PAGE 9 18. The best definition for and explanation of electronegativity is: a) Electronegativity is the measure of the tendency of a combined atom to attract a shared pair of electrons to itself; elements in the upper right hand side of the periodic table tend to be more ...
... REVIEW PROBLEMS FOR FINAL: PAGE 9 18. The best definition for and explanation of electronegativity is: a) Electronegativity is the measure of the tendency of a combined atom to attract a shared pair of electrons to itself; elements in the upper right hand side of the periodic table tend to be more ...
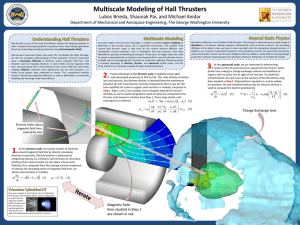
Lubos Brieda, Shaunak Pai, and Michael Keidar
... orbit. Instead of burning propellant to produce force, these devices generates thrust by accelerating ionized gas (plasma) using electromagnetic fields. Hall thrusters have been flown since early 70s. Yet despite this flight heritage, we still lack complete understanding of their operation. Primary ...
... orbit. Instead of burning propellant to produce force, these devices generates thrust by accelerating ionized gas (plasma) using electromagnetic fields. Hall thrusters have been flown since early 70s. Yet despite this flight heritage, we still lack complete understanding of their operation. Primary ...
Semester 1 Final Exam
... 18. What do the elements phosphorous and sulfur have in common? (A) They are both alkali metals. (B) They are in the same family. (C) They are in the same period. (D) They both form ions with the same charges. 19. Which of the following is a liquid non-metal atom at room temperature? (A) Barium (B) ...
... 18. What do the elements phosphorous and sulfur have in common? (A) They are both alkali metals. (B) They are in the same family. (C) They are in the same period. (D) They both form ions with the same charges. 19. Which of the following is a liquid non-metal atom at room temperature? (A) Barium (B) ...
Net ionic equation
... • Strong electrolytes: (solute is all ions) • Weak electrolytes: (some ions, mostly molecules) • Non-electrolytes: (no ions, all molecules) ...
... • Strong electrolytes: (solute is all ions) • Weak electrolytes: (some ions, mostly molecules) • Non-electrolytes: (no ions, all molecules) ...
Chapter 1 Introduction to Electricity
... Electric field – the space around a charged object in which another charged object experiences an electric force. Objects in this field can be attracted or repelled depending on their own charge. ...
... Electric field – the space around a charged object in which another charged object experiences an electric force. Objects in this field can be attracted or repelled depending on their own charge. ...
Chapter 2 Chemical Bonds Ionic Bonds
... Note: In the structure on the right, all atoms have a formal charge of 0 and the S atom is surrounded by 12 electrons in an expanded octet. Because it has the lowest formal charges, we expect the structure on the right to be the most favored one energetically and the one to make the greatest contrib ...
... Note: In the structure on the right, all atoms have a formal charge of 0 and the S atom is surrounded by 12 electrons in an expanded octet. Because it has the lowest formal charges, we expect the structure on the right to be the most favored one energetically and the one to make the greatest contrib ...
Redox Reactions: Transferring Electrons
... Because electrons are being transferred, the number of electrons gained must equal the number of electrons lost. Reducing Agent: the one that does the reducing. Oxidizing Agent: the one that does the oxidizing. Be Careful Here! Oxidation States: Electron Bookkeeping How do we know when a redox react ...
... Because electrons are being transferred, the number of electrons gained must equal the number of electrons lost. Reducing Agent: the one that does the reducing. Oxidizing Agent: the one that does the oxidizing. Be Careful Here! Oxidation States: Electron Bookkeeping How do we know when a redox react ...
Notes - Organization of Matter
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
First Semester complete review with answers
... o Noble gases are nonreactive (inert) because their valence energy level is full o Elements are generally reactive when the valence energy level is not full o Highly reactive = valence energy level is almost full, or the valence energy level is almost empty Understand how to read chemical formulas ...
... o Noble gases are nonreactive (inert) because their valence energy level is full o Elements are generally reactive when the valence energy level is not full o Highly reactive = valence energy level is almost full, or the valence energy level is almost empty Understand how to read chemical formulas ...
J.j. Thomson Atomic Theory — www.tutorvista.com — Readability
... Introduction to J.J.Thomson atomic theory:Thomson discovered the electron in the year 1897. His work put forward a new theory, that atom was made up of small particles.Thus he discovered the electrons. He proved his theory using the cathode ray tube. Scientists had already done many experiments to f ...
... Introduction to J.J.Thomson atomic theory:Thomson discovered the electron in the year 1897. His work put forward a new theory, that atom was made up of small particles.Thus he discovered the electrons. He proved his theory using the cathode ray tube. Scientists had already done many experiments to f ...
Metals
... A molecule is the smallest uncharged individual unit of a compound formed by two or more atoms. ...
... A molecule is the smallest uncharged individual unit of a compound formed by two or more atoms. ...
document
... You need to keep track of how you are doing in the class and take action if you fall behind or have trouble with the material. A. Fellow students - meet others in the class. Even though you and the other student may be perplexed about a subject, you will find that talking together in the language of ...
... You need to keep track of how you are doing in the class and take action if you fall behind or have trouble with the material. A. Fellow students - meet others in the class. Even though you and the other student may be perplexed about a subject, you will find that talking together in the language of ...
File
... A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. ...
... A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. ...
Page | 1 MATS1101 Chemistry notes semester 2 2012 TOPIC 1
... Ions: charged particle (atom or group of atoms)→when an atom gains or loses electrons it acquires a net charged, forms an ion Anions: negatively charged species (e.g. CL-, NO3-) no. protons < no. electrons Cations: Positively charged species (e.g. Na+, NH4+) no. protons > no. electrons ...
... Ions: charged particle (atom or group of atoms)→when an atom gains or loses electrons it acquires a net charged, forms an ion Anions: negatively charged species (e.g. CL-, NO3-) no. protons < no. electrons Cations: Positively charged species (e.g. Na+, NH4+) no. protons > no. electrons ...
Hydrogen Bonds
... Matter: Elements and Compounds Matter is anything that occupies space and has ...
... Matter: Elements and Compounds Matter is anything that occupies space and has ...
Honors Chemistry
... In a certain atom, the first and second principal energy levels are completely filled, as is the 3s sublevel. There are three electrons in the 3p sublevel. a. How many electrons are there in the atom? b. Give the electron configuration of the atom. c. What is the element’s symbol? ...
... In a certain atom, the first and second principal energy levels are completely filled, as is the 3s sublevel. There are three electrons in the 3p sublevel. a. How many electrons are there in the atom? b. Give the electron configuration of the atom. c. What is the element’s symbol? ...
chapter_2_2007
... Atoms can gain or lose electrons to achieve a full outermost energy level. – Atoms with charge are called ions. – When an atom gives away an electron, it ends up with more protons than electrons and gains a positive charge; cation – When an atom accepts an electron, it ends up with more electrons th ...
... Atoms can gain or lose electrons to achieve a full outermost energy level. – Atoms with charge are called ions. – When an atom gives away an electron, it ends up with more protons than electrons and gains a positive charge; cation – When an atom accepts an electron, it ends up with more electrons th ...
Name
... Essential Standard 9f: Apply simple mathematical relationships to determine one quantity given the other two (including speed= distance x time, density = mass/volume, force = pressure x area, volume = area x height). ...
... Essential Standard 9f: Apply simple mathematical relationships to determine one quantity given the other two (including speed= distance x time, density = mass/volume, force = pressure x area, volume = area x height). ...