Chem I Review Part 1
... E. Ernest Rutherford 23. Rutherford's experiment with alpha particle scattering by gold foil established that A. protons are not evenly distributed throughout an atom. B. electrons have a negative charge. C. electrons have a positive charge. D. atoms are made of protons, neutrons, and electrons. E. ...
... E. Ernest Rutherford 23. Rutherford's experiment with alpha particle scattering by gold foil established that A. protons are not evenly distributed throughout an atom. B. electrons have a negative charge. C. electrons have a positive charge. D. atoms are made of protons, neutrons, and electrons. E. ...
Dynamics and particle uxes in atmospheric
... also demand operation in electronegative environments. For example, in the fast growing field of plasma medicine,7,8 oxygen, an electronegative gas, is often introduced as a source of reactive oxygen species (ROS). Water, another electronegative precursor, is also likely to be present in many biomed ...
... also demand operation in electronegative environments. For example, in the fast growing field of plasma medicine,7,8 oxygen, an electronegative gas, is often introduced as a source of reactive oxygen species (ROS). Water, another electronegative precursor, is also likely to be present in many biomed ...
chemistry basics note - bramalea2010-msmanning
... ________________and _______________. The _____________ table lists elements in order of increasing atomic number. Molecules are ____________________________________________________. Molecules can contain only one element (i.e _______) or may contain different types of elements (i.e. ____) Su ...
... ________________and _______________. The _____________ table lists elements in order of increasing atomic number. Molecules are ____________________________________________________. Molecules can contain only one element (i.e _______) or may contain different types of elements (i.e. ____) Su ...
Collision Theory
... Theories of Chemical Kinetics: Collision Theory • Before atoms/molecules/ions can react, they must first collide • An effective collision between two species puts enough energy to break key bonds • The activation energy (Ea) is the minimum energy that must be supplied by collisions to trigger a rea ...
... Theories of Chemical Kinetics: Collision Theory • Before atoms/molecules/ions can react, they must first collide • An effective collision between two species puts enough energy to break key bonds • The activation energy (Ea) is the minimum energy that must be supplied by collisions to trigger a rea ...
Physical chemistry exam, quiz, homework with Solution
... atom Schrödinger equation because (A) the Laplacian operator has its simplest form in spherical polar coordinates. (B) cartesian coordinates would give particle-in-a-box wavefunctions. (C) the Schrödinger equation is then separable into 3 ordinary differential equations. (D) otherwise the atomic o ...
... atom Schrödinger equation because (A) the Laplacian operator has its simplest form in spherical polar coordinates. (B) cartesian coordinates would give particle-in-a-box wavefunctions. (C) the Schrödinger equation is then separable into 3 ordinary differential equations. (D) otherwise the atomic o ...
Show all work – Homework 5 –
... 1. Transition metal oxides containing a metal ion with multiple oxidation states may be non-stoichiometric. The non-stoichiometric compound may accommodate this by a variety of ways, including cation vacancies, oxide ion (anion) vacancies, interstitial cations, or interstitial anions. Soft chemistry ...
... 1. Transition metal oxides containing a metal ion with multiple oxidation states may be non-stoichiometric. The non-stoichiometric compound may accommodate this by a variety of ways, including cation vacancies, oxide ion (anion) vacancies, interstitial cations, or interstitial anions. Soft chemistry ...
Chapter 4 Notes
... discrete amounts of energy • Electrons only lose energy when they move to a lower energy state ...
... discrete amounts of energy • Electrons only lose energy when they move to a lower energy state ...
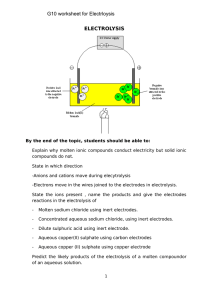
ELECTROLYSIS G10 worksheet for Electrloysis
... ________________________________________________________________________ ________________________________________________________________________ ...
... ________________________________________________________________________ ________________________________________________________________________ ...
Electron Configuration
... There are four types of sublevels—s,p,d and f. Inside the sublevels are atomic orbitals that hold the electrons. Every atomic orbital can hold two electrons. ...
... There are four types of sublevels—s,p,d and f. Inside the sublevels are atomic orbitals that hold the electrons. Every atomic orbital can hold two electrons. ...
Fall Exam 3
... Superimposing the electron density in a filled set of s, p and d orbitals results in a cubic distribution of electron density. ...
... Superimposing the electron density in a filled set of s, p and d orbitals results in a cubic distribution of electron density. ...
chapter 1 - Revsworld
... (18) A certain element has two naturally occurring isotopes. These isotopes have mass numbers of 63 and 65, and their fractional abundances are, respectively, 0.692 (69.2%) and 0.308 (30.8%). What is the atomic weight (or atomic mass) of this element? a) b) c.) d) e) ...
... (18) A certain element has two naturally occurring isotopes. These isotopes have mass numbers of 63 and 65, and their fractional abundances are, respectively, 0.692 (69.2%) and 0.308 (30.8%). What is the atomic weight (or atomic mass) of this element? a) b) c.) d) e) ...
Chapter 2 The Chemical Context of Life About 25 of the 92 natural
... A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) two more protons and two more neutrons than carbon-12. E) two more electrons and two more neutrons than carbon-12. Answer: C Topic: Concept 2.2 ...
... A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) two more protons and two more neutrons than carbon-12. E) two more electrons and two more neutrons than carbon-12. Answer: C Topic: Concept 2.2 ...
Chapter 4
... • Between elements in molecular compounds (remember two nonmetals make binary molecular compounds???) • Ionic - opposite charges (cations and anions) are attracted like magnets (strong bond) ...
... • Between elements in molecular compounds (remember two nonmetals make binary molecular compounds???) • Ionic - opposite charges (cations and anions) are attracted like magnets (strong bond) ...
Predicting Reactions • AP Chemistry CLASSIFYING REACTIONS
... are the reactions where the same chemical substance undergoes both oxidation and reduction. NO2 and H2O2 are classic examples: 3NO2(g) + H2O 2H+(aq) + 2NO3-(aq) + NO(g) 7. (Trick #1) During electrolysis of salts such as KI(aq), remember that K will not form in water; the water is reduced. If you do ...
... are the reactions where the same chemical substance undergoes both oxidation and reduction. NO2 and H2O2 are classic examples: 3NO2(g) + H2O 2H+(aq) + 2NO3-(aq) + NO(g) 7. (Trick #1) During electrolysis of salts such as KI(aq), remember that K will not form in water; the water is reduced. If you do ...
Table of Contents - Free Coursework for GCSE, IGCSE, A Level, IB
... When energy is applied to specific (individual) elements they emit a spectrum which only contains emissions of particular s. A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high ene ...
... When energy is applied to specific (individual) elements they emit a spectrum which only contains emissions of particular s. A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high ene ...
Atomic Theory
... When energy is applied to specific (individual) elements they emit a spectrum which only contains emissions of particular s. A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high ene ...
... When energy is applied to specific (individual) elements they emit a spectrum which only contains emissions of particular s. A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high ene ...
Safety - Wando High School
... 1. What makes a covalent bond? What makes an ionic bond? 2. What happens with the electrons in an ionic and covalent bond? 3. Why do atoms bond? 4. In a chemical formula what do the symbols and numbers represent? 5. What is a molecule? Is CO2 a molecule? Is NaCl a molecule? 6. What is an elements ox ...
... 1. What makes a covalent bond? What makes an ionic bond? 2. What happens with the electrons in an ionic and covalent bond? 3. Why do atoms bond? 4. In a chemical formula what do the symbols and numbers represent? 5. What is a molecule? Is CO2 a molecule? Is NaCl a molecule? 6. What is an elements ox ...
BH - hrsbstaff.ednet.ns.ca
... components freely move with each other. A solution is made from a solvent and a solute ...
... components freely move with each other. A solution is made from a solvent and a solute ...
CHEMISTRY 102B Name Hour Exam II March 19, 2015 Signature
... oxygen atom is lower than the 1st ionization energy for a nitrogen atom”? a) It is consistent with the general trend relating changes in ionization energy across a period from left to right because it is easier to take an electron from an oxygen atom than from a nitrogen atom. b) It is inconsistent ...
... oxygen atom is lower than the 1st ionization energy for a nitrogen atom”? a) It is consistent with the general trend relating changes in ionization energy across a period from left to right because it is easier to take an electron from an oxygen atom than from a nitrogen atom. b) It is inconsistent ...
Notes matter energy
... Atoms are mostly empty space. Protons and neutrons are located in a dense nucleus. Electrons occupy the space around the nucleus. The number electrons is equal to number of protons for neutral elements. Protons have a +1 electrical charge. Neutrons have no electrical charge. Electrons have a -1 elec ...
... Atoms are mostly empty space. Protons and neutrons are located in a dense nucleus. Electrons occupy the space around the nucleus. The number electrons is equal to number of protons for neutral elements. Protons have a +1 electrical charge. Neutrons have no electrical charge. Electrons have a -1 elec ...
Quantum Theory
... In this part of the Course (Part III), we shall look at matter “nanoscopically”, which deals with the basic elements of chemistry: atoms and molecules. Here, we shall first examine atoms - their physical properties and electronic structure - and the periodic table (Ch 9-10). Later, we shall examine ...
... In this part of the Course (Part III), we shall look at matter “nanoscopically”, which deals with the basic elements of chemistry: atoms and molecules. Here, we shall first examine atoms - their physical properties and electronic structure - and the periodic table (Ch 9-10). Later, we shall examine ...