Name
... Essential Standard 9f: Apply simple mathematical relationships to determine one quantity given the other two (including speed= distance x time, density = mass/volume, force = pressure x area, volume = area x height). ...
... Essential Standard 9f: Apply simple mathematical relationships to determine one quantity given the other two (including speed= distance x time, density = mass/volume, force = pressure x area, volume = area x height). ...
chapter_2_2009
... – There are two types of energy: – Potential energy Stored energy, available to do work – Kinetic energy Energy of motion – Potential energy can be converted to kinetic energy to do work. – A basic understanding of chemistry will help you to ...
... – There are two types of energy: – Potential energy Stored energy, available to do work – Kinetic energy Energy of motion – Potential energy can be converted to kinetic energy to do work. – A basic understanding of chemistry will help you to ...
ap chemistry unit two notes
... Mass conservation illustrated if number of each atom before and after reaction remains constant. Definite composition illustrated by formation of compounds that always have the same atom ratio. Different compounds made of same elements have small whole number ratios of those elements ...
... Mass conservation illustrated if number of each atom before and after reaction remains constant. Definite composition illustrated by formation of compounds that always have the same atom ratio. Different compounds made of same elements have small whole number ratios of those elements ...
Chemical Reactions Notes-1a-1
... All aqueous solutions can be classified in terms of whether or not they conduct electricity. ...
... All aqueous solutions can be classified in terms of whether or not they conduct electricity. ...
Practice exam - Dynamic Science
... Atoms from element “X” will give up some of their electrons. Element “X” will react with other element to form a gas. Element “X” is a very stable substance an will not react with other elements. ...
... Atoms from element “X” will give up some of their electrons. Element “X” will react with other element to form a gas. Element “X” is a very stable substance an will not react with other elements. ...
Chapter 2 2012
... atoms in a compound. The molecular formula of a compound specifies the number of each kind of atom present in a single molecular unit of a compound. • The number of atoms of each element is written as a subscript; when only a one atom of an element is present, the subscript is dropped. • In the case ...
... atoms in a compound. The molecular formula of a compound specifies the number of each kind of atom present in a single molecular unit of a compound. • The number of atoms of each element is written as a subscript; when only a one atom of an element is present, the subscript is dropped. • In the case ...
Cl -1
... 1. The oxidation number of any uncombined element is 0. 2. The oxidation number of a monatomic ion equals the charge on the ion. 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in ...
... 1. The oxidation number of any uncombined element is 0. 2. The oxidation number of a monatomic ion equals the charge on the ion. 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in ...
atomic number - geraldinescience
... • Based on similarities in their chemical properties, elements on the periodic table are arranged in columns, which are called groups. • An atom’s chemical properties are largely determined by the number of the outermost electrons in an atom’s electron cloud. These electrons are called valence elect ...
... • Based on similarities in their chemical properties, elements on the periodic table are arranged in columns, which are called groups. • An atom’s chemical properties are largely determined by the number of the outermost electrons in an atom’s electron cloud. These electrons are called valence elect ...
Chapter 2 Study Guides
... 13. The prefix mono-‐ means “one,” and the prefix poly-‐ means “many.” How are these meanings related to the terms monomer and polymer? ...
... 13. The prefix mono-‐ means “one,” and the prefix poly-‐ means “many.” How are these meanings related to the terms monomer and polymer? ...
IPC – First Semester Exam Review Be able to classify an example
... o Noble gases are nonreactive (inert) because their valence energy level is full o Elements are generally reactive when the valence energy level is not full o Highly reactive = valence energy level is almost full, or the valence energy level is almost empty Understand how to read chemical formulas ...
... o Noble gases are nonreactive (inert) because their valence energy level is full o Elements are generally reactive when the valence energy level is not full o Highly reactive = valence energy level is almost full, or the valence energy level is almost empty Understand how to read chemical formulas ...
Chem 1 Worksheets WSHEET 1: Working with Numbers Practice
... 2. Calculate the molar mass of Ca(BO2)2·6H2O. 3. Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide, Mg(OH)2. 4. Calculate the number of oxygen atoms in 29.34 g of sodium sulfate, Na2SO4. 5. Calculate the mass in grams of 8.35 1022 molecules of CBr4. 6. Household sugar, suc ...
... 2. Calculate the molar mass of Ca(BO2)2·6H2O. 3. Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide, Mg(OH)2. 4. Calculate the number of oxygen atoms in 29.34 g of sodium sulfate, Na2SO4. 5. Calculate the mass in grams of 8.35 1022 molecules of CBr4. 6. Household sugar, suc ...
Lone pairs
... Form pairs of two….quickly and quietly. Switch desks so that pairs are sitting next to each ...
... Form pairs of two….quickly and quietly. Switch desks so that pairs are sitting next to each ...
Balancing Redox Reactions 1 - VCC Library
... electrons lost in the oxidation process must equal the total number of electrons gained during the reduction process. In a redox reaction, the substance that gets oxidized (that loses electrons) is called the reducing agent because it reduces the other substance by giving its electrons. The substanc ...
... electrons lost in the oxidation process must equal the total number of electrons gained during the reduction process. In a redox reaction, the substance that gets oxidized (that loses electrons) is called the reducing agent because it reduces the other substance by giving its electrons. The substanc ...
The Periodic Table - Harlan Independent Schools
... All matter is composed of atoms and groups of atoms bonded together, called molecules. Substances that are made from one type of atom only are called pure substances. Substances that are made from more than one type of atom bonded together are called ...
... All matter is composed of atoms and groups of atoms bonded together, called molecules. Substances that are made from one type of atom only are called pure substances. Substances that are made from more than one type of atom bonded together are called ...
Electrochemistry I
... that we have two different solutions in direct contact with each other. This could probably be done by placing a felt divider (or some other barrier which will allow the movement of electricity, but not allow the solutions to mix) between the two solutions, but it would create problems. The Cu2+ and ...
... that we have two different solutions in direct contact with each other. This could probably be done by placing a felt divider (or some other barrier which will allow the movement of electricity, but not allow the solutions to mix) between the two solutions, but it would create problems. The Cu2+ and ...
File
... 28. Which element is a solid at STP and a good conductor strontium atom in an excited state? of electricity? A) 2–8–18–7–1 B) 2–8–18–7–3 A) iodine B) mercury C) 2–8–18–8–1 D) 2–8–18–8–2 19. Which electron configuration represents the electrons of C) nickel D) sulfur 29. Which statement explains why ...
... 28. Which element is a solid at STP and a good conductor strontium atom in an excited state? of electricity? A) 2–8–18–7–1 B) 2–8–18–7–3 A) iodine B) mercury C) 2–8–18–8–1 D) 2–8–18–8–2 19. Which electron configuration represents the electrons of C) nickel D) sulfur 29. Which statement explains why ...
Class Presentation – Naming and Formula Writing
... HW - Set C Key Set C Key: Formula to Name (Transition Metals) ...
... HW - Set C Key Set C Key: Formula to Name (Transition Metals) ...
Electronic Structure and Covalent Bonding
... Distribute pairs of electrons (pairs of dots) around each atom (except H) to give each atom eight electrons around it (the octet rule). If there are not enough electrons to give these atoms eight electrons, change single bonds between atoms to double or triple bonds by shifting non-bonded pairs ...
... Distribute pairs of electrons (pairs of dots) around each atom (except H) to give each atom eight electrons around it (the octet rule). If there are not enough electrons to give these atoms eight electrons, change single bonds between atoms to double or triple bonds by shifting non-bonded pairs ...
CH 101 Study Guide Test 2
... What is stoichiometry and why is it useful Convert moles of one compound to moles of another Identify conversion factors Convert grams of one compound to grams of another using a balanced equation Know what the limiting reactant , actual yield and theoretical yield are Know the formula for percent y ...
... What is stoichiometry and why is it useful Convert moles of one compound to moles of another Identify conversion factors Convert grams of one compound to grams of another using a balanced equation Know what the limiting reactant , actual yield and theoretical yield are Know the formula for percent y ...
AP Chemistry Summer Work
... [email protected] - feel free to email me with questions over the summer WELCOME to AP chemistry! The AP curriculum includes all of the topics and the labs that we need to complete before the 2015 AP test on Monday, May 4th. All of you will find AP chemistry to be challenging, some of you will find i ...
... [email protected] - feel free to email me with questions over the summer WELCOME to AP chemistry! The AP curriculum includes all of the topics and the labs that we need to complete before the 2015 AP test on Monday, May 4th. All of you will find AP chemistry to be challenging, some of you will find i ...
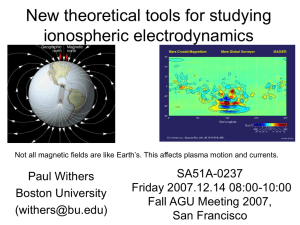
fallagu2007posterv02
... and collision frequencies) occurs below regions (F region) where ambipolar diffusion affects plasma number densities, so the theoretical inconsistencies are rarely noticed. However, the inconsistencies are present in most thermosphere-ionosphere-electrodynamics models and will affect the results of ...
... and collision frequencies) occurs below regions (F region) where ambipolar diffusion affects plasma number densities, so the theoretical inconsistencies are rarely noticed. However, the inconsistencies are present in most thermosphere-ionosphere-electrodynamics models and will affect the results of ...
New substances are formed by chemical reactions. When elements
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...