Unit 9 Study Guide - Hewlett
... a. mass – kg b. weight- Newtons c. gravity – 9.8 m/s2 d. energy (KE & PE) – Joules e. work – Joules f. power – Watts g. force – Newtons 3. Know the states of energy (Kinetic & Potential) and the forms of energy: a. thermal b. chemical c. mechanical d. electromagnetic (light) e. nuclear 4. Be able to ...
... a. mass – kg b. weight- Newtons c. gravity – 9.8 m/s2 d. energy (KE & PE) – Joules e. work – Joules f. power – Watts g. force – Newtons 3. Know the states of energy (Kinetic & Potential) and the forms of energy: a. thermal b. chemical c. mechanical d. electromagnetic (light) e. nuclear 4. Be able to ...
Types of Energy
... Types of Energy These are the two main types of energy: Kinetic: energy in motion Potential: stored energy The following forms of energy can be grouped into those two types. Heat (Thermal Energy): internal motion of molecules. Ex. water boiling, turning to steam. Mechanical: energy from motion and m ...
... Types of Energy These are the two main types of energy: Kinetic: energy in motion Potential: stored energy The following forms of energy can be grouped into those two types. Heat (Thermal Energy): internal motion of molecules. Ex. water boiling, turning to steam. Mechanical: energy from motion and m ...
Energy is a quantity that measures the ability to cause change in a
... • Batteries have energy because they can be used in a radio to make sound. • Gasoline has energy because it can be burned in an engine to move a car. ...
... • Batteries have energy because they can be used in a radio to make sound. • Gasoline has energy because it can be burned in an engine to move a car. ...
Relationships Between Heat and Work
... • As long as a substance does not change phase, its internal energy will increase as long as its temperature increases • Work can transfer energy to a substance – Results in an increase in internal energy • Can be transferred to another substance as heat ...
... • As long as a substance does not change phase, its internal energy will increase as long as its temperature increases • Work can transfer energy to a substance – Results in an increase in internal energy • Can be transferred to another substance as heat ...
Conservation of Energy Quiz
... 1. What form of energy is stored in any stretched or compressed object? 2. Is kinetic energy a vector quantity or a scalar quantity? 3. Describe the relationship between kinetic energy and gravitational potential energy during the free fall of a pencil from a desk. 4. A pocket watch contains a long, ...
... 1. What form of energy is stored in any stretched or compressed object? 2. Is kinetic energy a vector quantity or a scalar quantity? 3. Describe the relationship between kinetic energy and gravitational potential energy during the free fall of a pencil from a desk. 4. A pocket watch contains a long, ...
Energy
... • Also Travels in waves but are much slower than light • Is produced by vibrating air molecules which in turn vibrate our ear drums. ...
... • Also Travels in waves but are much slower than light • Is produced by vibrating air molecules which in turn vibrate our ear drums. ...
Verdana 30 pt
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
Slide 1
... “The energy in electromagnetic phenomena is the same as mechanical energy. The only question is, ‘Where does it reside?’ In the old theories, it resides in electrified bodies. In our theory, it resides in the electromagnetic field, in the space surrounding the electrified bodies.”—James Maxwell ...
... “The energy in electromagnetic phenomena is the same as mechanical energy. The only question is, ‘Where does it reside?’ In the old theories, it resides in electrified bodies. In our theory, it resides in the electromagnetic field, in the space surrounding the electrified bodies.”—James Maxwell ...
Verdana 30 pt - Liceo Statale Aprosio
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
Conservation of Energy Discussion (from 16.3) Here is a brief
... Here is a brief discussion of the origin of the term conservative for a vector field, F, that is the gradient of some potential function, f . Mathematically, this relationship is F = ∇f , but let’s see where the terms come from. FIRST, let F(x, y, z) be a force vector field that moves a particle of ...
... Here is a brief discussion of the origin of the term conservative for a vector field, F, that is the gradient of some potential function, f . Mathematically, this relationship is F = ∇f , but let’s see where the terms come from. FIRST, let F(x, y, z) be a force vector field that moves a particle of ...
Energy and Work
... greater position = more PE. Ex: Flowerpot on windowsill. The higher the windowsill, the greater PE so greater KE. ...
... greater position = more PE. Ex: Flowerpot on windowsill. The higher the windowsill, the greater PE so greater KE. ...
Temperature - Mwiseman.com
... • Were R is the ideal gas constant and T is temperature in Kelvin ...
... • Were R is the ideal gas constant and T is temperature in Kelvin ...
Topic 4-6 Socrative Quiz Answers
... 4. B – Evaporative Cooling 5. B - False – During a phase change the average energy remains the same 6. B - False – you would see a graph looking like steps because the temperature does not change during a phase change 7. D – Latent Heat 8. E – Sublimation 9. A – True – A substance always gains energ ...
... 4. B – Evaporative Cooling 5. B - False – During a phase change the average energy remains the same 6. B - False – you would see a graph looking like steps because the temperature does not change during a phase change 7. D – Latent Heat 8. E – Sublimation 9. A – True – A substance always gains energ ...
PPF
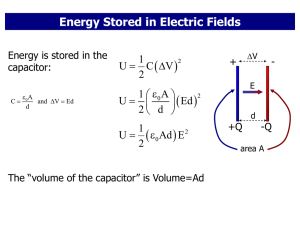
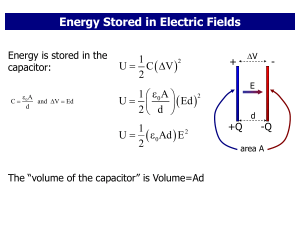
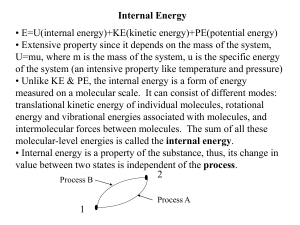
... • E=U(internal energy)+KE(kinetic energy)+PE(potential energy) • Extensive property since it depends on the mass of the system, U=mu, where m is the mass of the system, u is the specific energy of the system (an intensive property like temperature and pressure) • Unlike KE & PE, the internal energy ...
... • E=U(internal energy)+KE(kinetic energy)+PE(potential energy) • Extensive property since it depends on the mass of the system, U=mu, where m is the mass of the system, u is the specific energy of the system (an intensive property like temperature and pressure) • Unlike KE & PE, the internal energy ...
sgt2
... Drawing Free-body diagrams. Solving problems with frictional forces. Solving circular motion problems. Work-Energy theorem. Solving problems using conservation of energy principles. Hooke’s law. ...
... Drawing Free-body diagrams. Solving problems with frictional forces. Solving circular motion problems. Work-Energy theorem. Solving problems using conservation of energy principles. Hooke’s law. ...