Lecture 3 - McMaster Physics and Astronomy
... Fourth, heat is not a property of a system like temperature, pressure, volume or mass. It is energy in transit – energy that enters or leaves a system as a consequence of a temperature difference between the system and a body with which it is in thermal contact (including radiant heat). Neither work ...
... Fourth, heat is not a property of a system like temperature, pressure, volume or mass. It is energy in transit – energy that enters or leaves a system as a consequence of a temperature difference between the system and a body with which it is in thermal contact (including radiant heat). Neither work ...
Themodynamic notes section 6.1
... thermodynamic system is equal to the amount of heat energy (q) added to or lost by the system plus work done (w) on or by the system. DE = q + w • For work that only involves gas expansion or compression, w = -pDV; ...
... thermodynamic system is equal to the amount of heat energy (q) added to or lost by the system plus work done (w) on or by the system. DE = q + w • For work that only involves gas expansion or compression, w = -pDV; ...
Document
... The next step in discovering the law of conservation of energy was made by René Descartes, the French philosopher. He developed the idea that motion is conserved in all physical interactions. Descartes expressed motion by multiplying an object’s mass by its velocity. In contrast, the German philosop ...
... The next step in discovering the law of conservation of energy was made by René Descartes, the French philosopher. He developed the idea that motion is conserved in all physical interactions. Descartes expressed motion by multiplying an object’s mass by its velocity. In contrast, the German philosop ...
What is the relationship between kinetic and potential energy?
... What is the relationship between kinetic and potential energy? ...
... What is the relationship between kinetic and potential energy? ...
J S U
... 7. Write an expression for the work done when a force is acting on an object in the direction of its displacement . Define 1 J of work 8. What is the work to be done to increase the velocity of a car from 30 km/ h to 60 km/ h if the mass of the car is 1500 kg? 9. Define and find expression for kinet ...
... 7. Write an expression for the work done when a force is acting on an object in the direction of its displacement . Define 1 J of work 8. What is the work to be done to increase the velocity of a car from 30 km/ h to 60 km/ h if the mass of the car is 1500 kg? 9. Define and find expression for kinet ...
Handout Topic 2 Work , Energy, Power, Efficiency
... A mass falls near the Earth’s surface at constant speed in still air. Discuss the energy changes, if any, that occur in the gravitational potential energy and in the kinetic energy of the mass. ...
... A mass falls near the Earth’s surface at constant speed in still air. Discuss the energy changes, if any, that occur in the gravitational potential energy and in the kinetic energy of the mass. ...
Work and Energy - mrweaverphysics
... •Predict changes in mechanical energy when positive or negative work is done on the center of mass •Analyze a system and categorize the internal energy as potential, kinetic, or some combination of potential and kinetic. •Solve problems involving work, power, and/or energy •Solve problems involving ...
... •Predict changes in mechanical energy when positive or negative work is done on the center of mass •Analyze a system and categorize the internal energy as potential, kinetic, or some combination of potential and kinetic. •Solve problems involving work, power, and/or energy •Solve problems involving ...
Lecture_3 - Department of Mathematics
... kinetic energy E_kin exerts on a cubic container with volume V, (iii) combine this and the ideal gas law to show average molecular kinetic energy = 3kT/2 ...
... kinetic energy E_kin exerts on a cubic container with volume V, (iii) combine this and the ideal gas law to show average molecular kinetic energy = 3kT/2 ...
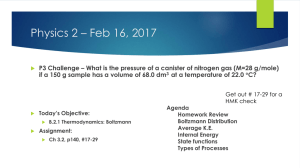
IB 3.2 Gases Feb 16 Agenda
... Problem solving is either of the plug and chug variety, or is algebra derivation of formulas types. So know these two fundamental relationships. (Only the first is in the IB packet.) ...
... Problem solving is either of the plug and chug variety, or is algebra derivation of formulas types. So know these two fundamental relationships. (Only the first is in the IB packet.) ...
Energy and Matter
... Energy Measurements – Calories (cal)= 4.184 J – BTU – Joules (J)- the work done when one kg is accelerated 1 m per second (1J= 1kg X m / s. – KWh ...
... Energy Measurements – Calories (cal)= 4.184 J – BTU – Joules (J)- the work done when one kg is accelerated 1 m per second (1J= 1kg X m / s. – KWh ...
Section 11
... An example of a system would be a flask with water and a balloon on the end of the flask. The flask is heated and the temperature of the water increases. Eventually increasing the volume of the balloon. ...
... An example of a system would be a flask with water and a balloon on the end of the flask. The flask is heated and the temperature of the water increases. Eventually increasing the volume of the balloon. ...
1 D.2. Energetic quantities: kinetic energy, work, total energy Force
... Remark. In (D9) W12 is the work on the path from the point (1) to the point (2). In 2D or 3D this work depends in general on the actual pathway. In the particular case when work does not depend on the actual path, one says the force is conservative, or that the force is given by a potential. Then an ...
... Remark. In (D9) W12 is the work on the path from the point (1) to the point (2). In 2D or 3D this work depends in general on the actual pathway. In the particular case when work does not depend on the actual path, one says the force is conservative, or that the force is given by a potential. Then an ...
Starter
... with a force of 10 N? 4. It takes 8 seconds for a pulley system to lift a load with 400 N.m. How much power is required? ...
... with a force of 10 N? 4. It takes 8 seconds for a pulley system to lift a load with 400 N.m. How much power is required? ...
Homework #13
... 1) Identify each of the terms in equation (6.4). How does this equation differ from that for a closed system? 2) Outline the circumstances which equation (6.4) reduces to the open system modified energy rate balance (MERB). 3) Under what circumstances can the kinetic energy terms in the open system ...
... 1) Identify each of the terms in equation (6.4). How does this equation differ from that for a closed system? 2) Outline the circumstances which equation (6.4) reduces to the open system modified energy rate balance (MERB). 3) Under what circumstances can the kinetic energy terms in the open system ...