appendix d - Florida Building Commission
... components, as defined in Section 3.1 for residential potable water heating. 2.2 Exclusion. This standard does not apply to desupereater/water heaters supplied as components of factory assembled refrigeration or air conditioning units. SECTION 3 DEFINITIONS 3.1 Desuperheater/water heater. A factory- ...
... components, as defined in Section 3.1 for residential potable water heating. 2.2 Exclusion. This standard does not apply to desupereater/water heaters supplied as components of factory assembled refrigeration or air conditioning units. SECTION 3 DEFINITIONS 3.1 Desuperheater/water heater. A factory- ...
Heating a house with gas
... R value is the thermal resistance of a material; that is how much is slows down the heat loss. U value is the reciprocal of the R value. U values cannot be added but R values can. The higher the R value the better, the lower the U value the better. The design temperature is the optimal temperature i ...
... R value is the thermal resistance of a material; that is how much is slows down the heat loss. U value is the reciprocal of the R value. U values cannot be added but R values can. The higher the R value the better, the lower the U value the better. The design temperature is the optimal temperature i ...
Document
... Imagine two systems A and B, separated by an adiabatic wall, while each is in contact with a third system C, via a conducting wall ]. The states of the systems change until both A and B come to thermal equilibrium with C. After this has happened if the adiabatic wall between A and B is replaced by a ...
... Imagine two systems A and B, separated by an adiabatic wall, while each is in contact with a third system C, via a conducting wall ]. The states of the systems change until both A and B come to thermal equilibrium with C. After this has happened if the adiabatic wall between A and B is replaced by a ...
Entropy, Carnot Engine and Thermoelectric Effect
... Thermodynamical Variables : Quantities which determine the state of system are known as thermodynamical variables. These include, temperature (T), pressure (P), volume (V), internal energy (U) and number of moles. ...
... Thermodynamical Variables : Quantities which determine the state of system are known as thermodynamical variables. These include, temperature (T), pressure (P), volume (V), internal energy (U) and number of moles. ...
10 Temperature and Heat
... An object at 0oC is hotter than an object at 0oF since 0oC is equivalent to 32oF. A 10 degree change in Celsius is a greater change in absolute temperature than a 10 degree change in Fahrenheit temperature. Celsius degrees are larger than Fahrenheit degrees. Yes. The change in volume will indicate a ...
... An object at 0oC is hotter than an object at 0oF since 0oC is equivalent to 32oF. A 10 degree change in Celsius is a greater change in absolute temperature than a 10 degree change in Fahrenheit temperature. Celsius degrees are larger than Fahrenheit degrees. Yes. The change in volume will indicate a ...
Practice Exam #2 with Answers
... _____17. Which of the following substances (with specific heat capacity provided) would show the greatest temperature change upon absorbing 100.0 J of heat? A) 10.0 g Fe, CFe = 0.449 J/g°C B) 10.0 g H2O, CH2O = 4.18 J/g°C C) 10.0 g ethanol, Cethanol = 2.42 J/g°C D) 10.0 g Au, CAu = 0.128 J/g°C ____ ...
... _____17. Which of the following substances (with specific heat capacity provided) would show the greatest temperature change upon absorbing 100.0 J of heat? A) 10.0 g Fe, CFe = 0.449 J/g°C B) 10.0 g H2O, CH2O = 4.18 J/g°C C) 10.0 g ethanol, Cethanol = 2.42 J/g°C D) 10.0 g Au, CAu = 0.128 J/g°C ____ ...
Practice ws on Ch 5 - mvhs
... 5. A 1.00 M solution of NaOH, a 1.00 M solution of HCl, and a calorimeter were allowed to stand at a room temperature of 19.5°C until the temperature of all three reached 19.5°C. A 200.0-mL sample of the 1.00 M NaOH was then placed in the calorimeter, 200.0 mL of the 1.00 M HCl was added as rapidly ...
... 5. A 1.00 M solution of NaOH, a 1.00 M solution of HCl, and a calorimeter were allowed to stand at a room temperature of 19.5°C until the temperature of all three reached 19.5°C. A 200.0-mL sample of the 1.00 M NaOH was then placed in the calorimeter, 200.0 mL of the 1.00 M HCl was added as rapidly ...
Cure Epoxies with Heat Heating Devices
... costs associated with providing heat is not consistent with industrywide objectives of lowering overall manufacturing costs. “Heat-curable epoxies are still widely available, but most companies are looking to decrease their overall costs to manufacture, especially time requirements,” claims Small. “ ...
... costs associated with providing heat is not consistent with industrywide objectives of lowering overall manufacturing costs. “Heat-curable epoxies are still widely available, but most companies are looking to decrease their overall costs to manufacture, especially time requirements,” claims Small. “ ...
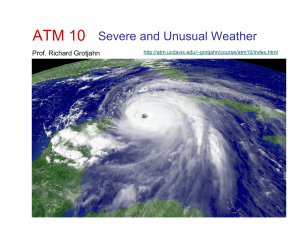
Lecture 2 - Richard Grotjahn
... Temperature celsius C Temperature Kelvin (use in eqns) K Temperature fahrenheit F Length meter m Time second s Note: do not use “centigrade” There are conversions between like quantity units T in K = T in C + 273, T in C = (T in F- 32)*(5/9) Ex: 275 K = 2 C + 273. 20 C = ( 68 F - 32)*(5/9) ...
... Temperature celsius C Temperature Kelvin (use in eqns) K Temperature fahrenheit F Length meter m Time second s Note: do not use “centigrade” There are conversions between like quantity units T in K = T in C + 273, T in C = (T in F- 32)*(5/9) Ex: 275 K = 2 C + 273. 20 C = ( 68 F - 32)*(5/9) ...
В диссертационной работе развиты и разработаны алгоритмы
... of matters both in the free and in dependent state. Advantage of such approach is in the following. If for the calculation of constituent of coefficient of heat conductivity to apply private derivatives, found from the system of equalizations of chemical equilibrium, on occasion there is possibility ...
... of matters both in the free and in dependent state. Advantage of such approach is in the following. If for the calculation of constituent of coefficient of heat conductivity to apply private derivatives, found from the system of equalizations of chemical equilibrium, on occasion there is possibility ...
Typical Specification for Heat Transfer Phoenix Evolution Guide
... gas with PVC Schedule 40 or 80 PVC or CPVC . The heater and all related gas piping shall be approved for zero clearance to any combustible surfaces. The heaters total combined equivalent venting length, including fitting allowances for both the intake air and exhaust gas shall not exceed 85' (in the ...
... gas with PVC Schedule 40 or 80 PVC or CPVC . The heater and all related gas piping shall be approved for zero clearance to any combustible surfaces. The heaters total combined equivalent venting length, including fitting allowances for both the intake air and exhaust gas shall not exceed 85' (in the ...
Introduction to Thermal Circuits for Steady-State, One
... Thermal conduction and convection are often phenomenologically introduced in firstsemester optomechanical courses. Respective expressions governing the relation between heat flux, in W/ m 2 , to either a linear material’s temperature gradient (conduction) or the difference in surface and ambient tem ...
... Thermal conduction and convection are often phenomenologically introduced in firstsemester optomechanical courses. Respective expressions governing the relation between heat flux, in W/ m 2 , to either a linear material’s temperature gradient (conduction) or the difference in surface and ambient tem ...
Convection Currents and the Mantle
... object. There are three ways in which heat can be transferred; conduction, convection and/or radiation. Click on the links below and answer the questions that follow. ...
... object. There are three ways in which heat can be transferred; conduction, convection and/or radiation. Click on the links below and answer the questions that follow. ...
Notes
... Energy can be defined as the capacity to do work or to transfer heat. Matter possesses energy both by virtue of its motion (kinetic energy) and by virtue of its position (potential energy). Thermochemistry is the study of the transfer of energy from one sample of matter to another in the form of hea ...
... Energy can be defined as the capacity to do work or to transfer heat. Matter possesses energy both by virtue of its motion (kinetic energy) and by virtue of its position (potential energy). Thermochemistry is the study of the transfer of energy from one sample of matter to another in the form of hea ...
calorimetry
... 1. Mass an unknown metal sample in a dry, previously massed 200-mm test tube. Place the test tube in a 400-mL beaker filled with water well above the level of the metal in the test tube. Heat to boiling and maintain this temperature for at least 5 minutes so that the metal reaches thermal equilibriu ...
... 1. Mass an unknown metal sample in a dry, previously massed 200-mm test tube. Place the test tube in a 400-mL beaker filled with water well above the level of the metal in the test tube. Heat to boiling and maintain this temperature for at least 5 minutes so that the metal reaches thermal equilibriu ...
Customer Application Brief General Industrial Filtration in Process
... To reduce contamination problems, 3M Purification Inc. recommends the use of cartridge filtration in the cooling water loop in the 15 µm - 25 µm range to reduce contamination problems and equipment fouling or damage. The 3M™ High Flow Series filter system is ideally suited to provide effective filtr ...
... To reduce contamination problems, 3M Purification Inc. recommends the use of cartridge filtration in the cooling water loop in the 15 µm - 25 µm range to reduce contamination problems and equipment fouling or damage. The 3M™ High Flow Series filter system is ideally suited to provide effective filtr ...
file
... What is the molar enthalpy of CO2 (g) in the reaction for the burning of butane below? 2 C4H10 +13 O2 8 CO2 +10 H2O ∆H=-5315 kJ Answer: Molar enthalpy is the enthalpy change in equation divided by the balance of CO2 Molar enthalpy, ∆H substance = 5315 kJ ÷ 8 ...
... What is the molar enthalpy of CO2 (g) in the reaction for the burning of butane below? 2 C4H10 +13 O2 8 CO2 +10 H2O ∆H=-5315 kJ Answer: Molar enthalpy is the enthalpy change in equation divided by the balance of CO2 Molar enthalpy, ∆H substance = 5315 kJ ÷ 8 ...
thermochemistry - Pace University Webspace
... certain amount of solvent. • H(soln) = H(soln) – H(components) ...
... certain amount of solvent. • H(soln) = H(soln) – H(components) ...
Energy and Heat Transfer
... Release of latent heat during cloud formation drives many atmospheric processes ...
... Release of latent heat during cloud formation drives many atmospheric processes ...
Hess's Law
... AP CHEM NOTES - Chapter 6 - THERMO (THERMOCHEMISTRY OR THERMODYNAMICS) Chemical Reactivity What drives chemical reactions? THERMODYNAMICS How do they occur? KINETICS ...
... AP CHEM NOTES - Chapter 6 - THERMO (THERMOCHEMISTRY OR THERMODYNAMICS) Chemical Reactivity What drives chemical reactions? THERMODYNAMICS How do they occur? KINETICS ...
Thermodynamics Temperature Scales Thermal Expansion and Stress
... Refrigerator and air conditions remove heat from the cold reservoir and put it into the surroundings (hot reservoir), keeping the food/room cold. A heat pump takes energy from the cold reservoir and puts it into a room or house (hot reservoir), thereby warming it. In either case, energy must be adde ...
... Refrigerator and air conditions remove heat from the cold reservoir and put it into the surroundings (hot reservoir), keeping the food/room cold. A heat pump takes energy from the cold reservoir and puts it into a room or house (hot reservoir), thereby warming it. In either case, energy must be adde ...
12.1 Thermodynamic Systems, States, and Processes 12.3
... system must change, (b) heat must be transferred from the system, (c) the internal energy of the system must change and/or heat must be transferred from the system, (d) heat must be transferred to the system. (c) MC When heat is added to a system of ideal gas during an isothermal expansion process, ...
... system must change, (b) heat must be transferred from the system, (c) the internal energy of the system must change and/or heat must be transferred from the system, (d) heat must be transferred to the system. (c) MC When heat is added to a system of ideal gas during an isothermal expansion process, ...