* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download file
Passive solar building design wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Dynamic insulation wikipedia , lookup
Solar water heating wikipedia , lookup
Solar air conditioning wikipedia , lookup
Intercooler wikipedia , lookup
Building insulation materials wikipedia , lookup
Heat exchanger wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Thermal conduction wikipedia , lookup
Cogeneration wikipedia , lookup
Heat equation wikipedia , lookup
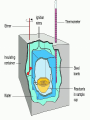
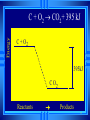
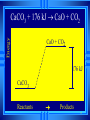
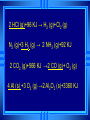
Calorimetry Calorimetry Calorimetry - the accurate and precise measurement of heat change for chemical and physical processes. The device used to measure the absorption or release of heat in chemical or physical processes is called a Calorimeter Calorimetry Foam cups are excellent heat insulators, and are commonly used as simple calorimeters A Cheap Calorimeter For systems at constant pressure, the heat content is the same as a property called Enthalpy (H) of the system Calorimetry in enthalpy = H q = H These terms will be used interchangeably in this textbook Thus, q = H = m x C x T H is negative for an exothermic reaction H is positive for an endothermic reaction Changes Calorimetry Calorimetry experiments can be performed at a constant volume using a device called a “bomb calorimeter” - a closed system In terms of bonds C O O O C O Breaking this bond will require energy. O C O C O O Making these bonds gives you energy. In this case making the bonds gives you more energy than breaking them. 9 Exothermic The products are lower in energy than the reactants Releases energy 2Al (s) + 3Cl2 (g) --> 2 AlCl3 (s) + 1408 kJ ∆H=1408 kJ 10 Energy C + O2 CO2+ 395 kJ C + O2 395kJ C O2 Reactants Products 11 Endothermic The products are higher in energy than the reactants Absorbs energy 2 H2O + 575 kJ ------> 2 H2 + 1 O2 (g) ∆H = + 572 kJ 12 Energy CaCO CaO CaCO CaO + CO+2 CO2 3 + 176 3 kJ CaO + CO2 176 kJ CaCO3 Reactants Products 13 Chemistry Happens in MOLES An equation that includes energy is called a thermochemical equation CH4 + 2O2 CO2 + 2H2O + 802.2 kJ 1 mole of CH4 releases 802.2 kJ of energy. When you make 802.2 kJ you also make 2 moles of water 14 What is the molar enthalpy of CO2 (g) in the reaction for the burning of butane below? 2 C4H10 +13 O2 8 CO2 +10 H2O ∆H=-5315 kJ Answer: Molar enthalpy is the enthalpy change in equation divided by the balance of CO2 Molar enthalpy, ∆H substance = 5315 kJ ÷ 8 mol = 664 kJ / mol. For each of the following rewrite the equation in " H " notation, for one mole of the underlined substance. Fe2O3 (s)+3CO(g)→3CO2(g)+2Fe(s)+25kJ Answer: 1/3 Fe2O3 (s)+CO(g)CO2(g)+2/3 Fe(s) ∆H = - 8.3 KJ 4 NH3(g)+5O2 (g)→4 NO(g)+6H2O(l)+1170kJ 2 HCl (g)+96 KJ → H2 (g)+Cl2 (g) N2 (g)+3 H2 (g) → 2 NH3 (g)+92 KJ 2 CO2 (g)+566 KJ →2 CO (g)+ O2 (g) 4 Al (s) +3 O2 (g) →2 Al2O3 (s)+3360 KJ Thermochemical Equations A heat of reaction is the heat change for the equation, exactly as written • The physical state of reactants and products must also be given. • Standard conditions for the reaction is 101.3 kPa (1 atm.) and 25 oC 18 CH4 + 2 O2 CO2 + 2 H2O + 802.2 kJ If 10. 3 grams of CH4 are burned completely, how much heat will be produced? 10. 3 g CH4 1 mol CH4 16.05 g CH4 802.2 kJ 1 mol CH4 = 514 kJ 19 CH4 + 2 O2 CO2 + 2 H2O + 802.2 kJ How many liters of O2 at STP would be required to produce 23 kJ of heat? How many grams of water would be produced with 506 kJ of heat? 20 How much heat will be released if 65 grams of butane is burned in a lighter according the equation: 2 C4H10 +13 O2 8 CO2 +10 H2O ∆H=-5315 kJ 1molC4 H10 5315kJ 65 gC4 H10 58.14 g 2molC4 H10 = 2976.4 kJ = 3.0 MJ Calculate the heat released when 120 grams of Iron (III) oxide is formed by the following equation 2 Fe2O3 (s) → 4 Fe(s)+3 O2 (g) ∆H=1625 kJ 1molFe2O3 1625kJ 120 gFe2O3 159.70 g 2mol = 610.5 kJ = 610 kJ Q = n ∆H (substance) Where n = # of moles What mass of carbon dioxide must form to create 1200 kJ of heat when the following reaction occurs? C6H12O6(s)+6O2(g)→6CO2(g)+6H2O(l) ∆H=- 2808kJ Answer: 110 grams 3) What mass of oxygen is needed to completely react and release 550 kJ of heat in the following reaction? 4Fe (s)+3O2 (g) → 2 Fe2O3 (s) ∆H=- 1625 kJ Answer: 32 grams Summary, so far... Enthalpy The heat content a substance has at a given temperature and pressure Can’t be measured directly because there is no set starting point The reactants start with a heat content The products end up with a heat content So we can measure how much enthalpy changes 27 Enthalpy Symbol is H Change in enthalpy is H (delta H) If heat is released, the heat content of the products is lower H is negative (exothermic) If heat is absorbed, the heat content of the products is higher H is positive (endothermic) 28 Energy Change is down H is <0 Reactants Products 29 Energy Change is up H is > 0 Reactants Products 30 Heat of Reaction The heat that is released or absorbed in a chemical reaction Equivalent to H C + O2(g) CO2(g) + 393.5 kJ C + O2(g) CO2(g) H = -393.5 kJ In thermochemical equation, it is important to indicate the physical state H2(g) + 1/2O2 (g) H2O(g) H = -241.8 kJ H2(g) + 1/2O2 (g) H2O(l) H = -285.8 kJ 31 Heat of Combustion The heat from the reaction that completely burns 1 mole of a substance 32 OBJECTIVES: • Classify, by type, the heat changes that occur during melting, freezing, boiling, and condensing. 33 OBJECTIVES: • Calculate heat changes that occur during melting, freezing, boiling, and condensing. 34 Heats of Fusion and Solidification Molar Heat of Fusion (Hfus) - the heat absorbed by one mole of a substance in melting from a solid to a liquid Molar Heat of Solidification (Hsolid) - heat lost when one mole of liquid solidifies 35 Heats of Fusion and Solidification Heat absorbed by a melting solid is equal to heat lost when a liquid solidifies • Thus, Hfus = -Hsolid 36 Heats of Vaporization and Condensation When liquids absorb heat at their boiling points, they become vapors. Molar Heat of Vaporization (Hvap) the amount of heat necessary to vaporize one mole of a given liquid. 37 Heats of Vaporization and Condensation Condensation is the opposite of vaporization. Molar Heat of Condensation (Hcond) - amount of heat released when one mole of vapor condenses Hvap = - Hcond 38 Heats of Vaporization and Condensation The large values for Hvap and Hcond are the reason hot vapors such as steam is very dangerous • You can receive a scalding burn from steam when the heat of condensation is released! 39 Heats of Vaporization and Condensation H20(g) H20(l) Hcond = - 40.7kJ/mol 40 Heat of Solution Heat changes can also occur when a solute dissolves in a solvent. Molar Heat of Solution (Hsoln) heat change caused by dissolution of one mole of substance Sodium hydroxide provides a good example of an exothermic molar heat of solution: 41 Heat of Solution NaOH(s) H2O(l) Na1+(aq) + OH1-(aq) Hsoln = - 445.1 kJ/mol The heat is released as the ions separate and interact with water, releasing 445.1 kJ of heat as Hsoln thus becoming so hot it steams 42