NH3 Cu NH3 NH3 H3N - Wilson`s Disease Support Group (UK)
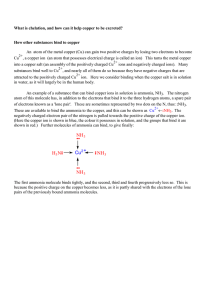
... Chelation and Wilson's disease Dr John Walshe found an unusual substance in the urine of patients who were being treated with penicillin, which led him to an impressive series of conclusions. Penicillamine, a breakdown product of penicillin, is an amino acid. Amino acids are the building blocks of p ...
... Chelation and Wilson's disease Dr John Walshe found an unusual substance in the urine of patients who were being treated with penicillin, which led him to an impressive series of conclusions. Penicillamine, a breakdown product of penicillin, is an amino acid. Amino acids are the building blocks of p ...
8.3 Temperature and Heat Heat Transfer Heat Flow
... heat flow occurs. • Convection – involves the movement of heated mass, more efficient than conduction for long distances. • Radiation – occurs even through a vacuum, depends on the properties of the radiating surface. ...
... heat flow occurs. • Convection – involves the movement of heated mass, more efficient than conduction for long distances. • Radiation – occurs even through a vacuum, depends on the properties of the radiating surface. ...
Set 3
... change the macroscopic configuration of the system. This in general can be undone. Consider the compression of an ideal gas by a piston. Of the system is adiabatically isolated, then by “easing up” on the piston, I allow the gas to return the energy to the piston. We usually think of the configurati ...
... change the macroscopic configuration of the system. This in general can be undone. Consider the compression of an ideal gas by a piston. Of the system is adiabatically isolated, then by “easing up” on the piston, I allow the gas to return the energy to the piston. We usually think of the configurati ...
Thermodynamics
... In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, ...
... In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, ...
First Law of Thermodynamics
... Irreversible process is one in which thermal system’s changes cannot be retraced, such as gas expanding to fill a vacuum through an open stopcock. A thermodynamic system can transfer its internal energy by changing the temperature (or phase) of another system of it can use its internal energy to do ...
... Irreversible process is one in which thermal system’s changes cannot be retraced, such as gas expanding to fill a vacuum through an open stopcock. A thermodynamic system can transfer its internal energy by changing the temperature (or phase) of another system of it can use its internal energy to do ...
The Heat Is On — High-Power Surface-Mount Resistors
... Conduction is the predominant means of heat transfer and thus many manufacturers will provide a thermal resistance for their device. Thermal resistance gives a first order approximation of how well the device transfers heat to the board and traces as power is applied, i.e. °C/W. This rating lacks ma ...
... Conduction is the predominant means of heat transfer and thus many manufacturers will provide a thermal resistance for their device. Thermal resistance gives a first order approximation of how well the device transfers heat to the board and traces as power is applied, i.e. °C/W. This rating lacks ma ...
... at peak hours. Solar peak hours can be defined as the period when radiation would be the dominant source of heating (Memon et al., 2009). As shown in different construction materials, granite showed the highest difference between surface and ambient temperature while the solar radiation depicted at ...
Document
... • Example – Phase change • Calculate the amount of energy needed to convert 55.0 grams of ice to all liquid water at its normal melting point. • Using the same amount of water calculate the energy needed to completely vaporize the water at its normal boiling point. ...
... • Example – Phase change • Calculate the amount of energy needed to convert 55.0 grams of ice to all liquid water at its normal melting point. • Using the same amount of water calculate the energy needed to completely vaporize the water at its normal boiling point. ...
Heat and Thermodynamics
... adiabatic processes as well as this application to heat engines. This ratio g = 1.66 for an ideal monoatomic gas and g = 1.4 for air, which is predominantly a diatomic gas. ...
... adiabatic processes as well as this application to heat engines. This ratio g = 1.66 for an ideal monoatomic gas and g = 1.4 for air, which is predominantly a diatomic gas. ...
Summary - Clarkson University
... "Thermodynamics is a funny subject. The first time you go through it, you don't understand it at all. The second time you go through it, you think you understand it, except for one or two small points. The third time you go through it, you know you don't understand it, but by then you are so used to ...
... "Thermodynamics is a funny subject. The first time you go through it, you don't understand it at all. The second time you go through it, you think you understand it, except for one or two small points. The third time you go through it, you know you don't understand it, but by then you are so used to ...
2003 ME Graduate Student Conference
... a thermo-structural simulation for nano-scale and microscale structures which are thermally actuated. We consider four applications: thermal positioning of wave guides [1-2], thermal actuation of micro-scale switches [3], a novel thermally actuated mechanical memory system [4], and thermal actuation ...
... a thermo-structural simulation for nano-scale and microscale structures which are thermally actuated. We consider four applications: thermal positioning of wave guides [1-2], thermal actuation of micro-scale switches [3], a novel thermally actuated mechanical memory system [4], and thermal actuation ...
Convective heat transfer
... (after an equilibration time), the spatial distribution of temperatures (temperature field) in the conducting object does not change any further. Thus, all partial derivatives of temperature with respect to space may either be zero or have nonzero values, but all derivatives of temperature at any po ...
... (after an equilibration time), the spatial distribution of temperatures (temperature field) in the conducting object does not change any further. Thus, all partial derivatives of temperature with respect to space may either be zero or have nonzero values, but all derivatives of temperature at any po ...
Heat And Thermodynamics - Figure B
... Temperature Scale Conversion’: Temperature in one scale can be converted into another using C − 0 K − 273 F − 32 x − FP ...
... Temperature Scale Conversion’: Temperature in one scale can be converted into another using C − 0 K − 273 F − 32 x − FP ...
Example
... For an insulated rod of length L and cross section A with ends in thermal contact with heat reservoirs at TH and TC. The average power conducted (Pcond) is: ...
... For an insulated rod of length L and cross section A with ends in thermal contact with heat reservoirs at TH and TC. The average power conducted (Pcond) is: ...
Chapter 2 Safe and Smart Physical Activity
... Repeated use over time causes injury to eventually appear. Hyperflexion-hyper-too much, flexion-means to bend. Avoid exercises that bend joints too far. ...
... Repeated use over time causes injury to eventually appear. Hyperflexion-hyper-too much, flexion-means to bend. Avoid exercises that bend joints too far. ...
Practice Exam
... valve is opened, and steam flows through a turbine and into an evacuated tank (volume = 10 ft3). When the pressure in the tank equals the supply line pressure the valve is closed. The work output of the turbine during this process is 85 Btu/lbm of steam. Assuming the process to be adiabatic, find: ( ...
... valve is opened, and steam flows through a turbine and into an evacuated tank (volume = 10 ft3). When the pressure in the tank equals the supply line pressure the valve is closed. The work output of the turbine during this process is 85 Btu/lbm of steam. Assuming the process to be adiabatic, find: ( ...
Temperature, Heat, and Expansion
... Degrees are the same size as the Celsius degree Zero = absolute zero Absolute zero – lowest possible temperature, the temperature at which all motion stops. Absolute zero about = -273.15° C. ...
... Degrees are the same size as the Celsius degree Zero = absolute zero Absolute zero – lowest possible temperature, the temperature at which all motion stops. Absolute zero about = -273.15° C. ...
Calorimetry Lab
... C. What was the effect of changing the initial temperature of the copper? ___________ ___________________________________________________________________ ___________________________________________________________________ 4. Draw conclusions: The amount that the water’s temperature increases depends ...
... C. What was the effect of changing the initial temperature of the copper? ___________ ___________________________________________________________________ ___________________________________________________________________ 4. Draw conclusions: The amount that the water’s temperature increases depends ...
Concepts for specific heat
... This recovers the classical result. On the other hand for α ≫ 1, i.e. for high temperatures, the probabilities for Pn are vanishingly small for n ≥ 2 and P1 = 1. This we find ~2 π 2 hEn i ≈ E1 = 2mL2 In this case the specific heat becomes zero, in contrast to the result kB /2 for high temperatures. ...
... This recovers the classical result. On the other hand for α ≫ 1, i.e. for high temperatures, the probabilities for Pn are vanishingly small for n ≥ 2 and P1 = 1. This we find ~2 π 2 hEn i ≈ E1 = 2mL2 In this case the specific heat becomes zero, in contrast to the result kB /2 for high temperatures. ...
LATENT HEAT STORAGE SYSTEMS
... solar energy is intermittent, unpredictable, and available only during the day. Hence, its application requires efficient thermal energy storage so that the surplus heat collected during sunshine hours may be stored for later use during the night. Similar problems rise in heat recovery systems, wher ...
... solar energy is intermittent, unpredictable, and available only during the day. Hence, its application requires efficient thermal energy storage so that the surplus heat collected during sunshine hours may be stored for later use during the night. Similar problems rise in heat recovery systems, wher ...
5.1 THERMAL QUANTITIES
... co/d c/imates and composite c/imates. The design of floors in the wfous climates requires speck/ attention both for moderation of indoor temperatures and foot comfort. The prob/em of surface and interstitial condensation can be so/ved by good therm/ design. ...
... co/d c/imates and composite c/imates. The design of floors in the wfous climates requires speck/ attention both for moderation of indoor temperatures and foot comfort. The prob/em of surface and interstitial condensation can be so/ved by good therm/ design. ...























