Temperature Regulation
... lecture on thermoregulation appropriate for first- or second-year medical students, as well as graduate students. As a “hook” to hold student interest, it takes several examples from the cinema. The material can be easily covered in two 50-minute presentations, or condensed, as appropriate, into a s ...
... lecture on thermoregulation appropriate for first- or second-year medical students, as well as graduate students. As a “hook” to hold student interest, it takes several examples from the cinema. The material can be easily covered in two 50-minute presentations, or condensed, as appropriate, into a s ...
PDF
... to the heating and reliability of nanoscale transistors [1]. While the phonon Boltzmann Transport Equation has been used at such scales, its electronic Joule heating term, typically modeled as the dot product of the macroscopic electric field and current density [2], cannot account for the microscop ...
... to the heating and reliability of nanoscale transistors [1]. While the phonon Boltzmann Transport Equation has been used at such scales, its electronic Joule heating term, typically modeled as the dot product of the macroscopic electric field and current density [2], cannot account for the microscop ...
Lecture 32 - PhysicsGivesYouWings
... • Isobaric specific heat capacity is the amount of heat required to raise the temperature of one mole (or one kilogram) by one degree at constant pressure: ...
... • Isobaric specific heat capacity is the amount of heat required to raise the temperature of one mole (or one kilogram) by one degree at constant pressure: ...
Heat Transfer and Friction Characteristics in Turbulent
... near the acute corners cannot spread deep into the corner. These facts imply that the enhancement of turbulent heat and momentum transfer by the secondary flow is stronger near the obtuse corners than near the acute ones. The effects of these secondary flows on the mean streamwise velocity, U/Ub, an ...
... near the acute corners cannot spread deep into the corner. These facts imply that the enhancement of turbulent heat and momentum transfer by the secondary flow is stronger near the obtuse corners than near the acute ones. The effects of these secondary flows on the mean streamwise velocity, U/Ub, an ...
L14
... only involves two processes – adiabatic and isothermal – each of which are easy to manipulate analytically. And, since it is the most efficient engine that can operate between a set of bounds, it is a useful constraint on engines in general. So we start with a set amount of gas in a piston of initia ...
... only involves two processes – adiabatic and isothermal – each of which are easy to manipulate analytically. And, since it is the most efficient engine that can operate between a set of bounds, it is a useful constraint on engines in general. So we start with a set amount of gas in a piston of initia ...
Chapter 11 Homework
... heat is given off when a substance freezes (heat of solidification) or condenses (heat of condensation). how to calculate total heat changes following a heating or cooling curve. Honors: how to calculate heat of reaction using standard enthalpy of formation (Hfo). ...
... heat is given off when a substance freezes (heat of solidification) or condenses (heat of condensation). how to calculate total heat changes following a heating or cooling curve. Honors: how to calculate heat of reaction using standard enthalpy of formation (Hfo). ...
1 CHAPTER 1 INTRODUCTORY REMARKS 1.1 Introduction
... Intensive quantities do not depend on the amount of material. Temperature and pressure are examples. Another would be the specific heat capacity of a substance, which is the amount of heat required to raise unit mass of it through one degree, and it might be expressed in J kg−1 Co −1. This is what i ...
... Intensive quantities do not depend on the amount of material. Temperature and pressure are examples. Another would be the specific heat capacity of a substance, which is the amount of heat required to raise unit mass of it through one degree, and it might be expressed in J kg−1 Co −1. This is what i ...
MEP 365 THERMAL ENGINEERING MEASUREMENTS (3: 2, 3)
... CLO_5 Students will be able to derive heat diffusion equation for steady 1-D conduction. CLO_6: Students will be able to solve steady 1-D basic heat conduction problems (plane walls, cylinders, spheres, composite walls, conduction with internal heat generation and extended surfaces). ...
... CLO_5 Students will be able to derive heat diffusion equation for steady 1-D conduction. CLO_6: Students will be able to solve steady 1-D basic heat conduction problems (plane walls, cylinders, spheres, composite walls, conduction with internal heat generation and extended surfaces). ...
Thermodynamics
... idealized as the mass of the system, the pressure of the system, and the volume of the system, or some other equivalent set of numbers." Thermodynamics, then, is concerned with several properties of matter; foremost among these is heat. Heat is energy transferred between substances or systems due to ...
... idealized as the mass of the system, the pressure of the system, and the volume of the system, or some other equivalent set of numbers." Thermodynamics, then, is concerned with several properties of matter; foremost among these is heat. Heat is energy transferred between substances or systems due to ...
Unit 09 - Midland ISD
... increases by 69.5 °C when 24,500 J are applied. The specific heat of liquid water is 4.18 J/g x °C. What is the mass of the sample of water? 4. When 34,700 J of heat are applied to a 350 g sample of an unknown material the temperature rises from 22.0°C to 173.0°C. What must be the specific heat of t ...
... increases by 69.5 °C when 24,500 J are applied. The specific heat of liquid water is 4.18 J/g x °C. What is the mass of the sample of water? 4. When 34,700 J of heat are applied to a 350 g sample of an unknown material the temperature rises from 22.0°C to 173.0°C. What must be the specific heat of t ...
SMALLER iS SMARTER!
... industry standard by making the copper tubing smaller and smarter. The result is an air conditioner or heat pump unit that can offer high efficiency, use less refrigerant, and deliver money-saving and energy-saving comfort to homeowners for years and years. Goodman’s SmartCoil™ coil is our most adva ...
... industry standard by making the copper tubing smaller and smarter. The result is an air conditioner or heat pump unit that can offer high efficiency, use less refrigerant, and deliver money-saving and energy-saving comfort to homeowners for years and years. Goodman’s SmartCoil™ coil is our most adva ...
Influence of the ambient temperature during heat pipe
... heat pipe were made of the same materials and used the same number and types of process materials, nevertheless be the heat pipes had the different transport capabilities. One possible cause could be a different temperature during filling and exhausting heat pipes. This assumption is confirmed by ex ...
... heat pipe were made of the same materials and used the same number and types of process materials, nevertheless be the heat pipes had the different transport capabilities. One possible cause could be a different temperature during filling and exhausting heat pipes. This assumption is confirmed by ex ...
Vėsinimo apkrovos skaičiavimas
... CLTD (cooling load temperature difference), SCL (solar cooling load factor), and CLF (cooling load factor): all include the effect of (1) time-lag in conductive heat gain through opaque exterior surfaces and (2) time delay by thermal storage in converting radiant heat gain to cooling load. a. CLTD i ...
... CLTD (cooling load temperature difference), SCL (solar cooling load factor), and CLF (cooling load factor): all include the effect of (1) time-lag in conductive heat gain through opaque exterior surfaces and (2) time delay by thermal storage in converting radiant heat gain to cooling load. a. CLTD i ...
52 research about the influence of internal heat gains on energy
... Also, according to the document submitted to public review, Romania's Energy Strategy, thermal energy consumption in Romanian industrial sector in 2008 was 323,490 thousand t.o.e. from 1795,490 thousand t.o.e., so 18% of the final consumption of thermal energy. In 2013, the thermal energy consumptio ...
... Also, according to the document submitted to public review, Romania's Energy Strategy, thermal energy consumption in Romanian industrial sector in 2008 was 323,490 thousand t.o.e. from 1795,490 thousand t.o.e., so 18% of the final consumption of thermal energy. In 2013, the thermal energy consumptio ...
finite volume analysis of convective heat transfer augmentation from
... The extended surfaces(fins) are frequently used in heat exchanging devices for the purpose of increasing the heat transfer between a primary surface and the surrounding fluid. E.A.M.Elshafei[1] performed the experiment on natural convection heat transfer from circular pin fin heat sinks subject to t ...
... The extended surfaces(fins) are frequently used in heat exchanging devices for the purpose of increasing the heat transfer between a primary surface and the surrounding fluid. E.A.M.Elshafei[1] performed the experiment on natural convection heat transfer from circular pin fin heat sinks subject to t ...
Conceptual Summary/Outline of Topics
... ii. Conduction: collisions of molecules with adjacent molecules (good and poor conductors) iii. Convection (really a subclass of conduction). 1. Fluid (air, water, magma) in contact with hot surface 2. Heat transfer to interface layer of fluid by conduction, followed by bulk motion carrying heated f ...
... ii. Conduction: collisions of molecules with adjacent molecules (good and poor conductors) iii. Convection (really a subclass of conduction). 1. Fluid (air, water, magma) in contact with hot surface 2. Heat transfer to interface layer of fluid by conduction, followed by bulk motion carrying heated f ...
First Law of Thermodynamics
... Here, we develop the principles of thermodynamics for a discrete system, namely, an air parcel moving through the circulation. A thermodynamic system can transfer its internal energy by changing the temperature (or phase) of another system of it can use its internal energy to do mechanical work on i ...
... Here, we develop the principles of thermodynamics for a discrete system, namely, an air parcel moving through the circulation. A thermodynamic system can transfer its internal energy by changing the temperature (or phase) of another system of it can use its internal energy to do mechanical work on i ...
Thermochemistry: Energy Flow and Chemical
... Comparing ∆E and ∆H: • Reactions that do not involve gases: Because liquids and solid undergo very small volume changes, ∆V = 0; thus P∆V = 0 and ∆H ≈ ∆E • Reactions in which the amount (mol) of gas does not change: when the total amount of gaseous reactants equals the total amount of gaseous produc ...
... Comparing ∆E and ∆H: • Reactions that do not involve gases: Because liquids and solid undergo very small volume changes, ∆V = 0; thus P∆V = 0 and ∆H ≈ ∆E • Reactions in which the amount (mol) of gas does not change: when the total amount of gaseous reactants equals the total amount of gaseous produc ...
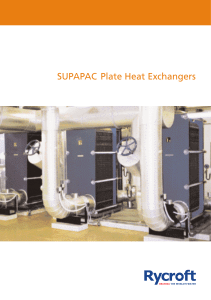
SUPAPAC Plate Heat Exchangers
... The exchangers comprise of pressed stainless steel ribbed plates vacuum brazed under carefully controlled conditions. The exchanger design has been developed using the latest computer simulation techniques backed up by extensive test bed trials. the net result is an exchanger whose reliability is un ...
... The exchangers comprise of pressed stainless steel ribbed plates vacuum brazed under carefully controlled conditions. The exchanger design has been developed using the latest computer simulation techniques backed up by extensive test bed trials. the net result is an exchanger whose reliability is un ...
Thermodynamics
... of entropy is that it appears to depend on a path variable. But the fact that the heat transfer isn't just any type of heat transfer, but only along a special kind of path, allows it to be a state variable. Put another way, calculating changes in entropy from one state to another, we must construct ...
... of entropy is that it appears to depend on a path variable. But the fact that the heat transfer isn't just any type of heat transfer, but only along a special kind of path, allows it to be a state variable. Put another way, calculating changes in entropy from one state to another, we must construct ...
CHAPTER I
... on the system and its internal energy is increased. If the piston moves out, then dV is positive, so W is positive and the system does work on its environment and its internal energy is reduced. This is a general expression of work for a gas, it isn’t piston and cylinder specific. For example, in a ...
... on the system and its internal energy is increased. If the piston moves out, then dV is positive, so W is positive and the system does work on its environment and its internal energy is reduced. This is a general expression of work for a gas, it isn’t piston and cylinder specific. For example, in a ...
Basic Thermodynamics Goals The ideal gas Entropy, Heat and Work
... gas slowly drawn into B by pulling out the piston B; piston A remains stationary. Show that the final temperature of the gas is Tf = Ti /22/3 . 9. In a free expansion of a perfect gas (also called a Joule expansion), we know U does not change, and no work is done. However, the entropy must increase ...
... gas slowly drawn into B by pulling out the piston B; piston A remains stationary. Show that the final temperature of the gas is Tf = Ti /22/3 . 9. In a free expansion of a perfect gas (also called a Joule expansion), we know U does not change, and no work is done. However, the entropy must increase ...
29-Thermal Exoskeleton - European School Luxembourg
... containing no free flowing charges, property for which it is a common material used in applications in contact with heat (in the following section, the tests carried out to prove the efficiency of this material are described also). ...
... containing no free flowing charges, property for which it is a common material used in applications in contact with heat (in the following section, the tests carried out to prove the efficiency of this material are described also). ...
Exam 1
... (R = 0.08206 L·atm/mol·K) 16. What volume of O2(g), measured at 17.7 °C and 0.978 atm reacts with 15.1 g C4H10 to produce CO2(g) and H2O(λ)? (R = 0.08206 L·atm/mol·K) 17. How many moles of helium are found in a balloon that contains 5.5 L of helium at a pressure of 1.15 atm and a temperature of 22.0 ...
... (R = 0.08206 L·atm/mol·K) 16. What volume of O2(g), measured at 17.7 °C and 0.978 atm reacts with 15.1 g C4H10 to produce CO2(g) and H2O(λ)? (R = 0.08206 L·atm/mol·K) 17. How many moles of helium are found in a balloon that contains 5.5 L of helium at a pressure of 1.15 atm and a temperature of 22.0 ...
The Second Law of Thermodynamics
... where, E is the Thompson violator, and R is a Carnot refrigerator. If we adjust the sizes of E and R such that all the work is used to run the Carnot refrigerator, and view the combined E-R apparatus as the system, we have succeeded in creating a device, which spontaneously pumps heat from cold to h ...
... where, E is the Thompson violator, and R is a Carnot refrigerator. If we adjust the sizes of E and R such that all the work is used to run the Carnot refrigerator, and view the combined E-R apparatus as the system, we have succeeded in creating a device, which spontaneously pumps heat from cold to h ...