Mock Exam One
... a.) The bond angle around the oxygen of an alcohol is < 109.5°. b.) Alcohols contain a polar covalent bond that an alkane does not have. c.) The strongest intermolecular force present in an alcohol is hydrogen bonding while the strongest intermolecular force present in alkanes is London dispersion. ...
... a.) The bond angle around the oxygen of an alcohol is < 109.5°. b.) Alcohols contain a polar covalent bond that an alkane does not have. c.) The strongest intermolecular force present in an alcohol is hydrogen bonding while the strongest intermolecular force present in alkanes is London dispersion. ...
Kinetic Assay of Human Pepsin with Albumin
... comparisons with results from other investigative centers. In general, research groups have tended to establish their own reference ranges for the given experiment, with the result that the reported values often do not have universal applicability. Moreover, no pure, stable human pepsin is yet avail ...
... comparisons with results from other investigative centers. In general, research groups have tended to establish their own reference ranges for the given experiment, with the result that the reported values often do not have universal applicability. Moreover, no pure, stable human pepsin is yet avail ...
Oxidation
... For calculating the oxidation state of carbon, we have to consider following two key things. ...
... For calculating the oxidation state of carbon, we have to consider following two key things. ...
MONOTONIC TUNING OF PLASMON RESONANCE USING
... Figure2. Micro- and nanofabrication of deformable nanoplasmonic membrane. Large area deformable nanoplasmonic membrane with uniform silver nanoislands were fabricated by using solid immersion metal dewetting (SIMD) and metal transfer to PDMS. Thin silver film was thermally evaporated on the thin flu ...
... Figure2. Micro- and nanofabrication of deformable nanoplasmonic membrane. Large area deformable nanoplasmonic membrane with uniform silver nanoislands were fabricated by using solid immersion metal dewetting (SIMD) and metal transfer to PDMS. Thin silver film was thermally evaporated on the thin flu ...
physical setting chemistry
... 9 An atom of carbon-12 and an atom of carbon-14 differ in (1) atomic number (2) mass number (3) nuclear charge (4) number of electrons ...
... 9 An atom of carbon-12 and an atom of carbon-14 differ in (1) atomic number (2) mass number (3) nuclear charge (4) number of electrons ...
Organic Chemistry Fifth Edition
... When the attacking atom is the same (oxygen in this case), nucleophilicity increases with increasing basicity. ...
... When the attacking atom is the same (oxygen in this case), nucleophilicity increases with increasing basicity. ...
File
... Directions: This test consists of two parts. Part 1 is multiple choice and Part 2 is constructed response. Write all responses in the spaces provided. Please use a pen. Submit ALL pages of this test (including any pages containing workings/outlines) to your supervising teacher upon completion. Part ...
... Directions: This test consists of two parts. Part 1 is multiple choice and Part 2 is constructed response. Write all responses in the spaces provided. Please use a pen. Submit ALL pages of this test (including any pages containing workings/outlines) to your supervising teacher upon completion. Part ...
Chapter 4
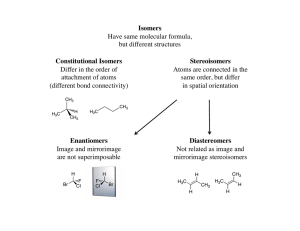
... Therefore if a given solution has 90% of one enantiomer (say R) and 10% of the other enantiomer (S) then the enantiomeric excess is 80% ...
... Therefore if a given solution has 90% of one enantiomer (say R) and 10% of the other enantiomer (S) then the enantiomeric excess is 80% ...
Full file at http://testbanksolution.eu/Test-Bank-Bank-for
... d. All resonance structures must have the same number of electrons ANS: A 50. Which of the following statements is not true about the carbonate anion, CO32? a. All of the oxygen atoms bear the same amount of charge b. All of the carbon-oxygen bonds are the same length c. The carbon atom bears the n ...
... d. All resonance structures must have the same number of electrons ANS: A 50. Which of the following statements is not true about the carbonate anion, CO32? a. All of the oxygen atoms bear the same amount of charge b. All of the carbon-oxygen bonds are the same length c. The carbon atom bears the n ...
2nd Semester Practice Chemistry Final 2009
... heat of gold is 0.13 J/g·ºC. a. 26 J c. 0.0006 J b. 26 J/g·ºC d. 0.0006 J/g·ºC 57. A system that changes spontaneously without an enthalpy change a. is impossible. c. becomes more disordered. b. becomes more ordered. d. releases heat. 58. What is the symbol for entropy? a. T c. G b. H d. S 59. What ...
... heat of gold is 0.13 J/g·ºC. a. 26 J c. 0.0006 J b. 26 J/g·ºC d. 0.0006 J/g·ºC 57. A system that changes spontaneously without an enthalpy change a. is impossible. c. becomes more disordered. b. becomes more ordered. d. releases heat. 58. What is the symbol for entropy? a. T c. G b. H d. S 59. What ...
PHYSICAL CHEMISTRY IN BRIEF
... The Physical Chemistry In Brief offers a digest of all major formulas, terms and definitions needed for an understanding of the subject. They are illustrated by schematic figures, simple worked-out examples, and a short accompanying text. The concept of the book makes it different from common univer ...
... The Physical Chemistry In Brief offers a digest of all major formulas, terms and definitions needed for an understanding of the subject. They are illustrated by schematic figures, simple worked-out examples, and a short accompanying text. The concept of the book makes it different from common univer ...
Chapter 18 pdf
... You have learned that some chemical systems have little tendency to react and others go readily to completion. In between these two extremes are the majority of reactions that reach a state of equilibrium with varying amounts of reactants unconsumed. If the reactants are not consumed, then not all t ...
... You have learned that some chemical systems have little tendency to react and others go readily to completion. In between these two extremes are the majority of reactions that reach a state of equilibrium with varying amounts of reactants unconsumed. If the reactants are not consumed, then not all t ...
THE SYNTHESIS AND CHARACTERIZATION OF PHOSPHONIUM INDENYLIDE COMPLEXES OF RUTHENIUM(II)
... found teaching that lab rewarding and I think I learned as much as my students did. There have been too many people whom I have crossed paths with to name that have made Queen’s the center of many fond memories. The regulars at Jay’s Bar and Grill, the Uehehe’s, Andrew Fraser, Jason Hanthorn, and Be ...
... found teaching that lab rewarding and I think I learned as much as my students did. There have been too many people whom I have crossed paths with to name that have made Queen’s the center of many fond memories. The regulars at Jay’s Bar and Grill, the Uehehe’s, Andrew Fraser, Jason Hanthorn, and Be ...
4420 Natural History of the Vertebrates. (3-3) Environmental
... 4425 Biometry. (3-3) Basic principles of statistical methods as applied to biological problems such as sampling techniques, analysis of data, experimental design and population dynamics. Emphasis will be on practical application. Prerequisites: BIO 2450 with a grade of “C” or higher; MATH 1315. 4426 ...
... 4425 Biometry. (3-3) Basic principles of statistical methods as applied to biological problems such as sampling techniques, analysis of data, experimental design and population dynamics. Emphasis will be on practical application. Prerequisites: BIO 2450 with a grade of “C” or higher; MATH 1315. 4426 ...
Stoichiometry
... Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. ...
... Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. ...
Barrier-free intermolecular proton transfer induced by excess
... Such a mechanism of a single-strand break has been computationally studied by Barrios et al., who suggested that only a small barrier would have to be overcome to create a sugarphosphate C–O bond rupture initiated by an excess electron attachment to a DNA base.6 Negatively charged clusters of biolog ...
... Such a mechanism of a single-strand break has been computationally studied by Barrios et al., who suggested that only a small barrier would have to be overcome to create a sugarphosphate C–O bond rupture initiated by an excess electron attachment to a DNA base.6 Negatively charged clusters of biolog ...
Anionic rearrangement of 2-benzyloxypyridine derivatives and a synthetic approach to aldingenin B
... Gregory Dudley again. As a young international graduate student, I joined his group with many questions about chemistry as well as the life in the United States. He was always accessible and helpful with all of my questions. More recently, we spoke about my future career plans: his advice, using his ...
... Gregory Dudley again. As a young international graduate student, I joined his group with many questions about chemistry as well as the life in the United States. He was always accessible and helpful with all of my questions. More recently, we spoke about my future career plans: his advice, using his ...