c8h18 isomers
... These intermolecular forces, which operate only over very small distances, result from induced polarization of the electron clouds in molecules. ♦ Within a family: The larger the molecule the stronger the intermolecular forces. ...
... These intermolecular forces, which operate only over very small distances, result from induced polarization of the electron clouds in molecules. ♦ Within a family: The larger the molecule the stronger the intermolecular forces. ...
NMR studies of the amyloid β -peptide
... degeneration and one that does not. The neurodegenerative misfolding diseases, in turn, consist of a wide variety of syndromes, including well-known diseases as Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), Huntington’s and Parkinson’s diseases. These diseases are so called neurodegener ...
... degeneration and one that does not. The neurodegenerative misfolding diseases, in turn, consist of a wide variety of syndromes, including well-known diseases as Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), Huntington’s and Parkinson’s diseases. These diseases are so called neurodegener ...
No Slide Title
... 25cm3 of 2.0M HCl was added to 25cm3 of 2.0M NaOH in an insulated beaker. The initial temperature of both solutions was 20°C. The highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. [The specific heat capacity (c) of water is 4.18 kJ K -1 kg -1] ...
... 25cm3 of 2.0M HCl was added to 25cm3 of 2.0M NaOH in an insulated beaker. The initial temperature of both solutions was 20°C. The highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. [The specific heat capacity (c) of water is 4.18 kJ K -1 kg -1] ...
Slide 1
... Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. ...
... Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • Interpretation: ratio of number of moles of reactant required to give the ratio of number of moles of product. • These ratios are called stoichiometric ratios. NB: Stoichiometric ratios are ideal proportions • Real ratios of reactants and products in the laboratory need to be measured (in grams an ...
... • Interpretation: ratio of number of moles of reactant required to give the ratio of number of moles of product. • These ratios are called stoichiometric ratios. NB: Stoichiometric ratios are ideal proportions • Real ratios of reactants and products in the laboratory need to be measured (in grams an ...
Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]
... some amino acids as secondary ligands, by NBS were studied. These reactions were found to proceed via a mechanism in which coordinated water was replaced by NBS prior to electron transfer step. Also, through bridging of NBS to the hydroxo conjugate species of the complex. Oxidation proceeds by homol ...
... some amino acids as secondary ligands, by NBS were studied. These reactions were found to proceed via a mechanism in which coordinated water was replaced by NBS prior to electron transfer step. Also, through bridging of NBS to the hydroxo conjugate species of the complex. Oxidation proceeds by homol ...
RUMPLE-DISSERTATION-2014 - SMARTech Home
... Eckert. The opportunity to work with such skilled scientists and kind mentors is a rare one, and I am extremely glad I had the opportunity to learn from them. I have always been in awe of their brilliance (I’m pretty sure they have each forgotten more than I’ll ever learn, and they still know so muc ...
... Eckert. The opportunity to work with such skilled scientists and kind mentors is a rare one, and I am extremely glad I had the opportunity to learn from them. I have always been in awe of their brilliance (I’m pretty sure they have each forgotten more than I’ll ever learn, and they still know so muc ...
2008 Equilibrium -- without math (PowerPoint 13 MB)
... right to left to reach equilibrium. • For reactions that have not reached equilibrium, such as the formation of HI considered above, we obtain the reaction quotient (Qc), instead of the equilibrium constant by substituting the initial concentrations into the equilibrium ...
... right to left to reach equilibrium. • For reactions that have not reached equilibrium, such as the formation of HI considered above, we obtain the reaction quotient (Qc), instead of the equilibrium constant by substituting the initial concentrations into the equilibrium ...
33 POLYMERS I OPTIONAL MODULE - 2
... linkages at all. The polymeric chains are held by weak VANDER WAAL forces and slide over one another. Due to lack of cross linkages these polymers soften on heating and harden or become rigid on cooling. Thus they can be moulded to any shape. Polythene, PVC, polystyrene are addition type thermoplast ...
... linkages at all. The polymeric chains are held by weak VANDER WAAL forces and slide over one another. Due to lack of cross linkages these polymers soften on heating and harden or become rigid on cooling. Thus they can be moulded to any shape. Polythene, PVC, polystyrene are addition type thermoplast ...
MEDICAL CHEMISTRY STUDY GUIDE
... Solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. This term is usually used to describe homogeneous mixtures of two or more liquids or of a liquid and one or more solids. Solutions may exist as gases, liquids, or solids. Nonreactive gases can mix in all pr ...
... Solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. This term is usually used to describe homogeneous mixtures of two or more liquids or of a liquid and one or more solids. Solutions may exist as gases, liquids, or solids. Nonreactive gases can mix in all pr ...
AS/A Level Chemistry (A) specimen question papers and mark
... Write your name, Centre number and candidate number in the spaces provided on the answer booklet. Write all your answers on the separate answer paper provided. If you use more than one sheet of paper, fasten the sheets together. Answer all questions. INFORMATION FOR CANDIDATES The number of marks is ...
... Write your name, Centre number and candidate number in the spaces provided on the answer booklet. Write all your answers on the separate answer paper provided. If you use more than one sheet of paper, fasten the sheets together. Answer all questions. INFORMATION FOR CANDIDATES The number of marks is ...
Equilibrium - pedagogics.ca
... to be shifted to the left, i.e. there is less ammonia present at equilibrium at the higher temperature. The effect of a temperature change on a system at equilibrium can be now considered in terms of Le Chatelier’s principle. Going back to the first reaction, the value of ∆H here refers to the forwa ...
... to be shifted to the left, i.e. there is less ammonia present at equilibrium at the higher temperature. The effect of a temperature change on a system at equilibrium can be now considered in terms of Le Chatelier’s principle. Going back to the first reaction, the value of ∆H here refers to the forwa ...
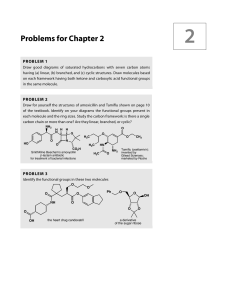
Problems for Chapter 2
... might again find it useful to draw a few structures to start with. (a) IR: 1745 cm–1; 13C NMR 214, 82, 58, and 41 ppm (b) IR: 3300 cm–1 (broad); 13C NMR 62 and 79 ppm. (c) IR: 1770 cm–1; 13C NMR 178, 86, 40, and 27 ppm. (d) IR: 1720 and 1650 cm –1 (strong); 13C NMR 165, 133, 131, and 54 ppm. ...
... might again find it useful to draw a few structures to start with. (a) IR: 1745 cm–1; 13C NMR 214, 82, 58, and 41 ppm (b) IR: 3300 cm–1 (broad); 13C NMR 62 and 79 ppm. (c) IR: 1770 cm–1; 13C NMR 178, 86, 40, and 27 ppm. (d) IR: 1720 and 1650 cm –1 (strong); 13C NMR 165, 133, 131, and 54 ppm. ...
Fundamentals of Environmental Chemistry
... assume—as many introductory chemistry books do somewhat awkwardly—that the reader knows nothing of the meaning of these terms. Chapter 2 discusses matter largely on the basis of its physical nature and behavior, introducing physical and chemical properties, states of matter, the mole as a quantity o ...
... assume—as many introductory chemistry books do somewhat awkwardly—that the reader knows nothing of the meaning of these terms. Chapter 2 discusses matter largely on the basis of its physical nature and behavior, introducing physical and chemical properties, states of matter, the mole as a quantity o ...
4.04 Nomenclature and Isomerism in Organic Chemistry
... rotation. An equimolar mixture of two optical isomers will thus have no effect on plane polarised light and is thus not optically active. Such mixtures are said to be racemic mixtures or racemates. A racemic mixture is an equimolar mixture of two optical isomers. Racemic mixtures are not optically a ...
... rotation. An equimolar mixture of two optical isomers will thus have no effect on plane polarised light and is thus not optically active. Such mixtures are said to be racemic mixtures or racemates. A racemic mixture is an equimolar mixture of two optical isomers. Racemic mixtures are not optically a ...
National German Competition and Problems of the IChO
... X is a colourless oil with a boiling point of 156 °C. It is used as starting material to produce different synthetic fibres such as Perlon or Dederon. Compound X consists of 73,43 % of carbon and 10,27 % of hyxdrogen. a) Complete the structural formulas of the intermediates A, B and the product X in ...
... X is a colourless oil with a boiling point of 156 °C. It is used as starting material to produce different synthetic fibres such as Perlon or Dederon. Compound X consists of 73,43 % of carbon and 10,27 % of hyxdrogen. a) Complete the structural formulas of the intermediates A, B and the product X in ...
General and Inorganic Chemistry – Laboratory Techniques
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... particularly for more advanced work. It is designed not only to encourage students to undertake special work but to aid them in later years in the solution of practical problems. No claim whatsoever is made for completeness. In their selection of material the authors have been guided simply by their ...
... particularly for more advanced work. It is designed not only to encourage students to undertake special work but to aid them in later years in the solution of practical problems. No claim whatsoever is made for completeness. In their selection of material the authors have been guided simply by their ...
Edita Pusvaškienė
... Since the carbon nanotube coating layer was not uniform (was thicker in the middle of the groove and thinner at the edges) the extraction of the less volatile compounds could be problematic because of their low sorption/desorption from the thicker coating layer and could cause peak tailing because ...
... Since the carbon nanotube coating layer was not uniform (was thicker in the middle of the groove and thinner at the edges) the extraction of the less volatile compounds could be problematic because of their low sorption/desorption from the thicker coating layer and could cause peak tailing because ...
Review on "Complex organic molecules at metal surfaces: bonding
... References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ...
... References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ...





![Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]](http://s1.studyres.com/store/data/008844767_1-9b02a033035d53dea970333df8a85c48-300x300.png)