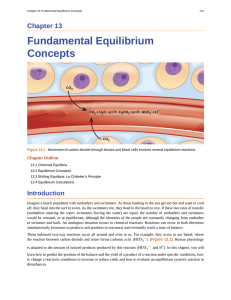
- Catalyst
... (b) How many grams of carbon are in 24.35 g of sucrose? (a) Determining the mass percent of each element: mass of C per mole sucrose = 12 x 12.01 g C/mol = 144.12 g C/mol mass of H / mol = 22 x 1.008 g H/mol = 22.176 g H/mol mass of O / mol = 11 x 16.00 g O/mol = 176.00 g O/mol total mass per mole = ...
... (b) How many grams of carbon are in 24.35 g of sucrose? (a) Determining the mass percent of each element: mass of C per mole sucrose = 12 x 12.01 g C/mol = 144.12 g C/mol mass of H / mol = 22 x 1.008 g H/mol = 22.176 g H/mol mass of O / mol = 11 x 16.00 g O/mol = 176.00 g O/mol total mass per mole = ...
Section – B - About iTutoring
... ions of second group are less in comparison to solubility products of sulphides of metal of III B group ions, therefore, HCl is added before adding H2S water to test the second group ions. H2S(aq) 2H+(aq) + S2-(aq) HCl(aq) H+(aq) + Cl-(aq) The common ion available from HCl creates common ion effect ...
... ions of second group are less in comparison to solubility products of sulphides of metal of III B group ions, therefore, HCl is added before adding H2S water to test the second group ions. H2S(aq) 2H+(aq) + S2-(aq) HCl(aq) H+(aq) + Cl-(aq) The common ion available from HCl creates common ion effect ...
Modification of Polycaprolactone by Titania Through a Sol
... PCL-g-AA/TiO2 hybrid also produced a broad O−H bond stretching at about 3000-3800cm−1 (Fig. 1D). This is due to the formation of hetero-associated hydrogen bonds between carboxylic acid groups of PCL-g-AA and the titanium bonded isopropyl group. In addition, acetate ligands of the PCL-g-AA/TiO2 hybr ...
... PCL-g-AA/TiO2 hybrid also produced a broad O−H bond stretching at about 3000-3800cm−1 (Fig. 1D). This is due to the formation of hetero-associated hydrogen bonds between carboxylic acid groups of PCL-g-AA and the titanium bonded isopropyl group. In addition, acetate ligands of the PCL-g-AA/TiO2 hybr ...
AQA Science GCSE Chemistry
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
Support Material
... Q.31. The electrical conductivity of a metal decreases with rise in temperature while that of a semi-conductor increases. Explain. Ans. In metals with increase of temperature, the kernels start vibrating at faster rate and thus offer resistance to the ow of electrons. Hence, conductivity decreases. ...
... Q.31. The electrical conductivity of a metal decreases with rise in temperature while that of a semi-conductor increases. Explain. Ans. In metals with increase of temperature, the kernels start vibrating at faster rate and thus offer resistance to the ow of electrons. Hence, conductivity decreases. ...
CHAPTER 9 Stoichiometry - Modern Chemistry Textbook
... reaction-stoichiometry calculations described in this chapter are theoretical. They tell us the amounts of reactants and products for a given chemical reaction under ideal conditions, in which all reactants are completely converted into products. However, ideal conditions are rarely met in the labor ...
... reaction-stoichiometry calculations described in this chapter are theoretical. They tell us the amounts of reactants and products for a given chemical reaction under ideal conditions, in which all reactants are completely converted into products. However, ideal conditions are rarely met in the labor ...
Concept based notes Chemistry Lab Manual
... auto catalyses the reaction & reaction proceed rapidly. Q. 29. What is the qualitative analysis? Ans. Qualitative analysis is used to separate and detect cations and anions in a sample substance. Q. 30. What is a radical? Ans. A radical may be defining as an atom which carries charges & behaves as a ...
... auto catalyses the reaction & reaction proceed rapidly. Q. 29. What is the qualitative analysis? Ans. Qualitative analysis is used to separate and detect cations and anions in a sample substance. Q. 30. What is a radical? Ans. A radical may be defining as an atom which carries charges & behaves as a ...
МЕТОДИЧЕСКИЕ УКАЗАНИЯ СТУДЕНТАМ
... 1. What volume (in mL) of 40% H3PO4 solution (ρ=1,25 g/cm3) is necessary to prepare 400mL of 0,25N of phosphoric acid solution (ρ=1g/cm3)? Calculate the mole fraction of H3PO4 in the obtained solution. 2. How many grams of Na2CO3·10H2O are necessary to prepare 100mL of 10% Na2CO3 solution (ρ=1,12 g/ ...
... 1. What volume (in mL) of 40% H3PO4 solution (ρ=1,25 g/cm3) is necessary to prepare 400mL of 0,25N of phosphoric acid solution (ρ=1g/cm3)? Calculate the mole fraction of H3PO4 in the obtained solution. 2. How many grams of Na2CO3·10H2O are necessary to prepare 100mL of 10% Na2CO3 solution (ρ=1,12 g/ ...
D--All Websites-eChemistryHelp-.mdi
... electrons lost or gained by an element during its change from free state into that compound or Oxidation number of an element in a particular compound represents the extent of oxidation or reduction of an element during its change from free state into that compound. 2. Oxidation number is given posi ...
... electrons lost or gained by an element during its change from free state into that compound or Oxidation number of an element in a particular compound represents the extent of oxidation or reduction of an element during its change from free state into that compound. 2. Oxidation number is given posi ...
5 How Chemists Measure Atoms and Molecules
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
FREE Sample Here
... A law is always given in the form of a mathematical expression. Once a hypothesis passes one or two tests it is considered a theory. Observation is a key component of the scientific method. ...
... A law is always given in the form of a mathematical expression. Once a hypothesis passes one or two tests it is considered a theory. Observation is a key component of the scientific method. ...
Forward
... Catalysis by crown ethers has been used to advantage to increase the rate of many organic reactions that involve anions as reactants. Just as important, though, is the increased understanding that studies of crown ether catalysis have brought to our knowledge of biological processes in which metal i ...
... Catalysis by crown ethers has been used to advantage to increase the rate of many organic reactions that involve anions as reactants. Just as important, though, is the increased understanding that studies of crown ether catalysis have brought to our knowledge of biological processes in which metal i ...
KCl + O KClO 3 → However, this equation is not balanced, since
... Writing a balanced chemical equation: In order to write a chemical equation, it is essential to know what substances are reacting and what substances are formed. It is only through experiment that a knowledge of what occurs can be discovered ─ either your experiment or the experiments of others (whi ...
... Writing a balanced chemical equation: In order to write a chemical equation, it is essential to know what substances are reacting and what substances are formed. It is only through experiment that a knowledge of what occurs can be discovered ─ either your experiment or the experiments of others (whi ...
5 How Chemists Measure Atoms and Molecules
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
1.09 MB / 64 pages
... (a) Vitamins that are soluble in the fatty tissues in our bodies will tend to stay in the body for a substantial period of time. Vitamins that are soluble in water will dissolve in fluids like our blood plasma and can be transported to the kidneys, removed from the blood stream, and excreted. The fa ...
... (a) Vitamins that are soluble in the fatty tissues in our bodies will tend to stay in the body for a substantial period of time. Vitamins that are soluble in water will dissolve in fluids like our blood plasma and can be transported to the kidneys, removed from the blood stream, and excreted. The fa ...
National German competition
... In alkaline solutions, p-nitrophenol is deeply yellow. In acid solutions, however, it has a pale greenish yellow colour. c) Explain the different colours of p-nitrophenol in an acid and in an alkaline solution. d) What are substances showing such an effect like p-nitrophenol used for? What advantage ...
... In alkaline solutions, p-nitrophenol is deeply yellow. In acid solutions, however, it has a pale greenish yellow colour. c) Explain the different colours of p-nitrophenol in an acid and in an alkaline solution. d) What are substances showing such an effect like p-nitrophenol used for? What advantage ...
Biochemistry - ChemGod.com
... They are big molecules. They tend to form solids due to a combination of Van der Waal’s forces and dipole forces. BUT, unsaturated molecules can be sterically hindered so that the polar parts can’t get near the other polar parts. That leaves us with just Van der Waal’s forces and it reduces the melt ...
... They are big molecules. They tend to form solids due to a combination of Van der Waal’s forces and dipole forces. BUT, unsaturated molecules can be sterically hindered so that the polar parts can’t get near the other polar parts. That leaves us with just Van der Waal’s forces and it reduces the melt ...
PDF File
... RNA. The most novel feature of this structure was the side-by-side packing of RNA helices, a feature not present in tRNA. Although many of the details have required revision, including the proposed tertiary interactions, this general feature of the model and the general disposition of helices in the ...
... RNA. The most novel feature of this structure was the side-by-side packing of RNA helices, a feature not present in tRNA. Although many of the details have required revision, including the proposed tertiary interactions, this general feature of the model and the general disposition of helices in the ...