Chapter 6
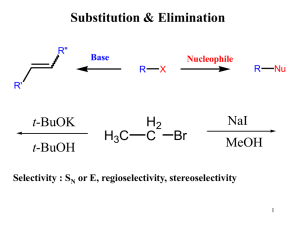
... • Methyl halides: only one C, CH3X • Primary: C to which X is bonded has only one C-C bond. • Secondary: C to which X is bonded has two C-C bonds. • Tertiary: C to which X is bonded has three C-C bonds. ...
... • Methyl halides: only one C, CH3X • Primary: C to which X is bonded has only one C-C bond. • Secondary: C to which X is bonded has two C-C bonds. • Tertiary: C to which X is bonded has three C-C bonds. ...
COMPETITION PTOBLEMS 1
... This publication contains the competition problems from the first twenty International Chemistry Olympiads (ICHO) organized in the years 1968 – 1988. It has been published by the ICHO International Information Centre in Bratislava (Slovakia) on the occasion of the 40th anniversary of this internatio ...
... This publication contains the competition problems from the first twenty International Chemistry Olympiads (ICHO) organized in the years 1968 – 1988. It has been published by the ICHO International Information Centre in Bratislava (Slovakia) on the occasion of the 40th anniversary of this internatio ...
iNTRODUCTiON TO ORGANiC COMPOUNDS
... Secondary and tertiary amines are named according to a “common” naming system. Primary amines can have either IUPAC or common names. Amines are the only organic compounds for which we will learn common names. In the common system, amines are named by adding the names of the alkyl group(s) attached t ...
... Secondary and tertiary amines are named according to a “common” naming system. Primary amines can have either IUPAC or common names. Amines are the only organic compounds for which we will learn common names. In the common system, amines are named by adding the names of the alkyl group(s) attached t ...
College Chemistry
... measurement were exact to the nearest 0.01 cm, it would have been recorded as 15.70 cm. We say that the first measurement is accurate to 3 significant figures and the second to 4. A recorded volume of 2.8 L represents two significant figures. If this same volume were written 0.028 m3, it would still ...
... measurement were exact to the nearest 0.01 cm, it would have been recorded as 15.70 cm. We say that the first measurement is accurate to 3 significant figures and the second to 4. A recorded volume of 2.8 L represents two significant figures. If this same volume were written 0.028 m3, it would still ...
Ch14_PT MULTIPLE CHOICE. Choose the one alternative that best
... 44) As the molar mass of these alcohols increases, the water solubility decreases. This occurs because the polarity of the hydroxyl group, which is the reason for the interaction with the polar water molecules, becomes less important as the size of the nonpolar hydrocarbon portion of the molecule in ...
... 44) As the molar mass of these alcohols increases, the water solubility decreases. This occurs because the polarity of the hydroxyl group, which is the reason for the interaction with the polar water molecules, becomes less important as the size of the nonpolar hydrocarbon portion of the molecule in ...
Alcohols, Phenols, and Ethers
... CH3 methyl t-butyl ether (MTBE) O A common gasoline additive used as an octane booster to oxygenate the gas, and ...
... CH3 methyl t-butyl ether (MTBE) O A common gasoline additive used as an octane booster to oxygenate the gas, and ...
Improved Synthesis of Seven-Coordinate Molybdenum( I I) and
... General Procedures. All reactions were carried out with the highest purity reagents commercially available. Molybdenum hexacarbonylwas obtained from Climax Molybdenum or Pressure Chemical Co., and tungsten .hexacarbonyl was obtained from Pressure Chemical Co. Isocyanides were prepared by standard pr ...
... General Procedures. All reactions were carried out with the highest purity reagents commercially available. Molybdenum hexacarbonylwas obtained from Climax Molybdenum or Pressure Chemical Co., and tungsten .hexacarbonyl was obtained from Pressure Chemical Co. Isocyanides were prepared by standard pr ...
Chemistry Final Exam Review
... ____ 25. Subscripts are used to balance chemical reactions. ____ 26. A synthesis reaction contains two products. ____ 27. A decomposition reaction contains at least two products. ____ 28. A combustion reaction will always yield water as a product. ____ 29. A single displacement reaction has two comp ...
... ____ 25. Subscripts are used to balance chemical reactions. ____ 26. A synthesis reaction contains two products. ____ 27. A decomposition reaction contains at least two products. ____ 28. A combustion reaction will always yield water as a product. ____ 29. A single displacement reaction has two comp ...
presentation source
... Carbon skeletons and hydrogen skins When determining the number of different skeletons, remember that: Each carbon forms a maximum of four single bonds, OR two single and one double bond, OR one single and triple bond. The arrangement of carbon atoms determines the skeleton, so a straight chain an ...
... Carbon skeletons and hydrogen skins When determining the number of different skeletons, remember that: Each carbon forms a maximum of four single bonds, OR two single and one double bond, OR one single and triple bond. The arrangement of carbon atoms determines the skeleton, so a straight chain an ...
Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]
... 1. Combination Reactions In combination reactions two or more substances react to form one product. For example, magnesium metal burns brilliantly in air to produce magnesium oxide: 2Mg(s) + O2(g) g 2 MgO(s) This reaction is used to produce the bright flame generated by flares and some fireworks. A ...
... 1. Combination Reactions In combination reactions two or more substances react to form one product. For example, magnesium metal burns brilliantly in air to produce magnesium oxide: 2Mg(s) + O2(g) g 2 MgO(s) This reaction is used to produce the bright flame generated by flares and some fireworks. A ...
Stoichiometry - Social Circle City Schools
... this is the reverse of the process that is used to determine percent composition. The first step in the process is to understand that the percent composition of any element is equal to the mass of that element in exactly 100 g of compound. For example, a compound containing only phosphorus and chlor ...
... this is the reverse of the process that is used to determine percent composition. The first step in the process is to understand that the percent composition of any element is equal to the mass of that element in exactly 100 g of compound. For example, a compound containing only phosphorus and chlor ...
Reduction of CuO and Cu2O with H2: H Embedding
... isothermal conditions were similar to the ones determined by TPR means (no presence of an intermediate phase). In addition, an induction period for reaction was observed as shown in Figure 1b. This figure represents the change for the intensity of the Cu2O phase as a function of time at isothermal c ...
... isothermal conditions were similar to the ones determined by TPR means (no presence of an intermediate phase). In addition, an induction period for reaction was observed as shown in Figure 1b. This figure represents the change for the intensity of the Cu2O phase as a function of time at isothermal c ...
free sample
... B) The term "strong electrolyte" means that the substance is extremely reactive. C) A strong acid solution consists of only partially ionized acid molecules. D) The term "weak electrolyte" means that the substance is inert. E) A molecular compound that does not ionize in solution is considered a str ...
... B) The term "strong electrolyte" means that the substance is extremely reactive. C) A strong acid solution consists of only partially ionized acid molecules. D) The term "weak electrolyte" means that the substance is inert. E) A molecular compound that does not ionize in solution is considered a str ...
Document
... One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl group to –oxy. Name the remaining alkyl group as an alkane, with the alkoxy group as a ...
... One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl group to –oxy. Name the remaining alkyl group as an alkane, with the alkoxy group as a ...
Stoichiometric Calculations
... equation in terms of particles (which could be molecules, atoms, or formula units). Multiplying the number of moles by 6.02 x 1023 yields the numbers of particles. So a formula that's balanced for moles must also be balanced for particles. can be read as: ...
... equation in terms of particles (which could be molecules, atoms, or formula units). Multiplying the number of moles by 6.02 x 1023 yields the numbers of particles. So a formula that's balanced for moles must also be balanced for particles. can be read as: ...
Class Notes
... 1. After reaction, the original carbonyl carbon will have one and only one C-O single bond 2. For formaldehyde, aldehydes, and ketones, one R group adds (reactions 4-6) 3. For esters or carbonyl chlorides (“acid chlorides”), two R groups add o Replace not only the carbonyl p-bond, but also the “extr ...
... 1. After reaction, the original carbonyl carbon will have one and only one C-O single bond 2. For formaldehyde, aldehydes, and ketones, one R group adds (reactions 4-6) 3. For esters or carbonyl chlorides (“acid chlorides”), two R groups add o Replace not only the carbonyl p-bond, but also the “extr ...









![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)