questions based on high order thinking skill - Entrance
... AgI crystallizes in a cubic closed packed ZnS structure. What fraction of tetrahedral site is occupied by Ag ion ? ...
... AgI crystallizes in a cubic closed packed ZnS structure. What fraction of tetrahedral site is occupied by Ag ion ? ...
questions based on high order thinking skill
... AgI crystallizes in a cubic closed packed ZnS structure. What fraction of tetrahedral site is occupied by Ag ion ? ...
... AgI crystallizes in a cubic closed packed ZnS structure. What fraction of tetrahedral site is occupied by Ag ion ? ...
Chem 12 SM Ch5 Review final new ok revised
... 24. Methane, gasoline, and propane are effective fuel sources because they are all hydrocarbons in which the molecules contain only C–H and C–C bonds. These are high energy bonds that will break and release a large amount of energy upon combustion. 25. We are able to rearrange two chemical equations ...
... 24. Methane, gasoline, and propane are effective fuel sources because they are all hydrocarbons in which the molecules contain only C–H and C–C bonds. These are high energy bonds that will break and release a large amount of energy upon combustion. 25. We are able to rearrange two chemical equations ...
Chapter 12
... What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
... What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
Alkenes and Alkynes I
... in the substrate will inhibit substitution) When a synthesis must begin with a primary alkyl halide, use a bulky base. (Because the steric bulk of the base will inhibit substitution) ...
... in the substrate will inhibit substitution) When a synthesis must begin with a primary alkyl halide, use a bulky base. (Because the steric bulk of the base will inhibit substitution) ...
Lorell Thesis Final Version in PDF S
... other men, living and dead, and that I must exert myself in order to give in the same measure as I have received and am still receiving. Albert Einstein ...
... other men, living and dead, and that I must exert myself in order to give in the same measure as I have received and am still receiving. Albert Einstein ...
Supplemental Problems
... living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its body no longer takes in new carbon-14. Thus, by measuring how much carbon-14 a once-living obj ...
... living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its body no longer takes in new carbon-14. Thus, by measuring how much carbon-14 a once-living obj ...
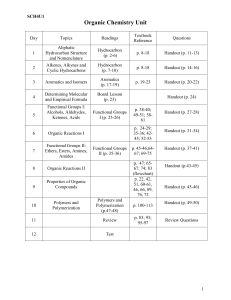
Organic Chemistry Package 2011
... This works for simple alkanes such as butane (C4H10) and pentane (C5H12). However, it becomes hopeless when larger alkanes are considered. For example there are 5 isomers of hexane (C6H14), 9 isomers of heptane (C7H16) and 75 isomers of decane (C10H22). Another problem arises as far as nomenclature ...
... This works for simple alkanes such as butane (C4H10) and pentane (C5H12). However, it becomes hopeless when larger alkanes are considered. For example there are 5 isomers of hexane (C6H14), 9 isomers of heptane (C7H16) and 75 isomers of decane (C10H22). Another problem arises as far as nomenclature ...
Course Notes
... Magnetic equivalence is usually the same as chemical equivalence. Equivalence can be established by symmetry operations such as rotation, mirror planes and centers of symmetry Chemically equivalent protons have the same chemical shifts. To determine if protons are chemically equivalent, replace one ...
... Magnetic equivalence is usually the same as chemical equivalence. Equivalence can be established by symmetry operations such as rotation, mirror planes and centers of symmetry Chemically equivalent protons have the same chemical shifts. To determine if protons are chemically equivalent, replace one ...
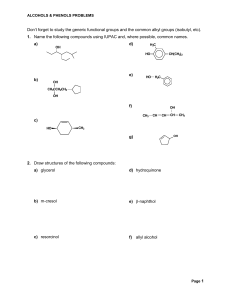
Don`t forget to study the generic functional groups and the common
... Also draw the structures of any carbonyl compounds that can be reduced by a LiAlH4 to ...
... Also draw the structures of any carbonyl compounds that can be reduced by a LiAlH4 to ...
Organic Chemistry/Fourth Edition: e-Text
... Ethyl isopropyl ketone may be alternatively named 2-methyl-3-pentanone. Its longest continuous chain has five carbons. The carbonyl carbon is C-3 irrespective of the direction in which the chain is numbered, and so we choose the direction that gives the lower number to the position that bears the me ...
... Ethyl isopropyl ketone may be alternatively named 2-methyl-3-pentanone. Its longest continuous chain has five carbons. The carbonyl carbon is C-3 irrespective of the direction in which the chain is numbered, and so we choose the direction that gives the lower number to the position that bears the me ...
Chapter 9
... • It contains 6.022 x 1023 atoms (Avogadro’s number) of the element. The molar mass of an element or compound is the sum of the atomic masses of all its atoms. of a substance ...
... • It contains 6.022 x 1023 atoms (Avogadro’s number) of the element. The molar mass of an element or compound is the sum of the atomic masses of all its atoms. of a substance ...
Part I Carbohydrate Auxiliaries - Wiley-VCH
... and highly diverse starting materials) and consequently the reactions follow different mechanisms. In one successful example Kunz employed his galactosylamine auxiliary as chiral template in the Ugi reaction (Scheme 1.9) [12]. When galactosylamine 3 was allowed to react with an aldehyde, an isocyani ...
... and highly diverse starting materials) and consequently the reactions follow different mechanisms. In one successful example Kunz employed his galactosylamine auxiliary as chiral template in the Ugi reaction (Scheme 1.9) [12]. When galactosylamine 3 was allowed to react with an aldehyde, an isocyani ...
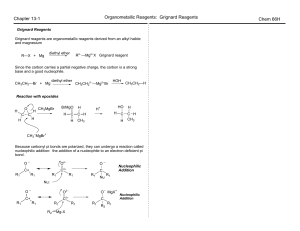
CH221 CLASS 13
... solubilize the alkene, and N-bromosuccinimide (NBS) is used as a (safer) supply of bromine. E.g. O ...
... solubilize the alkene, and N-bromosuccinimide (NBS) is used as a (safer) supply of bromine. E.g. O ...
Experimental details
... pressure as well as the very intense ionizing radiation emitted. The relevant materials in nuclear technological applications are required to be stable and durable after having withstood these extreme conditions. The materials stability under high temperature and pressure is primarily controlled by ...
... pressure as well as the very intense ionizing radiation emitted. The relevant materials in nuclear technological applications are required to be stable and durable after having withstood these extreme conditions. The materials stability under high temperature and pressure is primarily controlled by ...
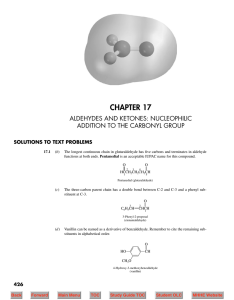
Document
... The Wittig Reaction • Since phosphorus is a second-row element, it can be surrounded by more than eight electrons. • Thus, a second resonance structure can be drawn that places a double bond between carbon and phosphorus. • Regardless of which resonance structure is drawn, a Wittig reagent has no ne ...
... The Wittig Reaction • Since phosphorus is a second-row element, it can be surrounded by more than eight electrons. • Thus, a second resonance structure can be drawn that places a double bond between carbon and phosphorus. • Regardless of which resonance structure is drawn, a Wittig reagent has no ne ...
Stoichiometry - Milton
... M uch of our knowledge of chemistry is based on the careful quantitative analysis of substances involved in chemical reactions. Composition stoichiometry (which you studied in Chapter 3) deals with the mass relationships of elements in compounds. Reaction stoichiometry involves the mass relationship ...
... M uch of our knowledge of chemistry is based on the careful quantitative analysis of substances involved in chemical reactions. Composition stoichiometry (which you studied in Chapter 3) deals with the mass relationships of elements in compounds. Reaction stoichiometry involves the mass relationship ...
Version PREVIEW – Exam 3 – JOHNSON – (53140) 1 This print
... The standard state of carbon is graphite; consequently, graphite’s standard free energy of formation is zero. Diamond is actually thermodynamically less stable than graphite; consequently, diamond’s reversion to graphite is spontaneous. First Law Thermo 01 002 5.0 points What is true about the first ...
... The standard state of carbon is graphite; consequently, graphite’s standard free energy of formation is zero. Diamond is actually thermodynamically less stable than graphite; consequently, diamond’s reversion to graphite is spontaneous. First Law Thermo 01 002 5.0 points What is true about the first ...
COMPARATIVE EVALUATION OF TCF BLEACHED
... production. During the past thirteen years a major part of the softwood has been replaced by hardwood species with the proportion of softM!ood shifting from 80% (in 1985) to only 46% (in 1998). It is expected that this trend will continue provided that hardwood high-purity pulps will meet the specif ...
... production. During the past thirteen years a major part of the softwood has been replaced by hardwood species with the proportion of softM!ood shifting from 80% (in 1985) to only 46% (in 1998). It is expected that this trend will continue provided that hardwood high-purity pulps will meet the specif ...