2009 Nov (9746) Paper 1
... (c) AgI is not fully ionic but has considerable percentage of covalent character because I – ion (0.216 nm) is much larger than F – ion (0.136 nm). Hence, I – anion is readily polarised by the Ag+ ion leading to electron density between the Ag+ and I – ions; i.e. electrons are incompletely transferr ...
... (c) AgI is not fully ionic but has considerable percentage of covalent character because I – ion (0.216 nm) is much larger than F – ion (0.136 nm). Hence, I – anion is readily polarised by the Ag+ ion leading to electron density between the Ag+ and I – ions; i.e. electrons are incompletely transferr ...
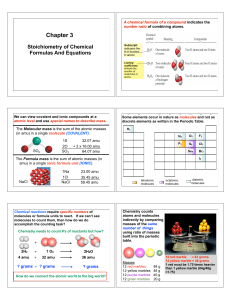
The Mole
... LIMITING REACTANT. The other reactant(s) are in EXCESS. To find the amount of excess, subtract the amount used from the given amount. If you have to find more than one product, be sure to start with the limiting reactant. You don’t have to determine which is the LR over and over again! ...
... LIMITING REACTANT. The other reactant(s) are in EXCESS. To find the amount of excess, subtract the amount used from the given amount. If you have to find more than one product, be sure to start with the limiting reactant. You don’t have to determine which is the LR over and over again! ...
Changing Matter
... • To show conservation of mass Balance equations – Make sure there are the same number of each type of atom in the products and in the reactants ...
... • To show conservation of mass Balance equations – Make sure there are the same number of each type of atom in the products and in the reactants ...
Fundamentals
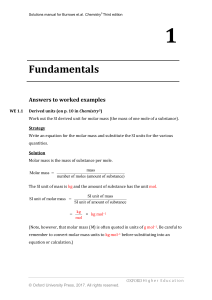
... Relative atomic mass of bromine (on p. 17 in Chemistry3) Naturally occurring bromine contains 79Br (abundance 50.69%) and 81Br (abundance 49.31%). Calculate the relative atomic mass of naturally occurring bromine. ...
... Relative atomic mass of bromine (on p. 17 in Chemistry3) Naturally occurring bromine contains 79Br (abundance 50.69%) and 81Br (abundance 49.31%). Calculate the relative atomic mass of naturally occurring bromine. ...
The Impact of Ligand Design on the Coordination Chemistry and
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
enthalpy changes
... The initial temperature of both solutions was 20°C. The highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. [The specific heat capacity (c) of water is 4.18 kJ oC -1 kg -1] ...
... The initial temperature of both solutions was 20°C. The highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. [The specific heat capacity (c) of water is 4.18 kJ oC -1 kg -1] ...
File
... Let's say the volume of the container is decreased. This increases the total pressure of the system. Increasing the pressure will increase the concentrations of all three species the same amount. Since there are more moles of gas (N2(g) 2(g)) on the left side, there is more "stuff" increased in the ...
... Let's say the volume of the container is decreased. This increases the total pressure of the system. Increasing the pressure will increase the concentrations of all three species the same amount. Since there are more moles of gas (N2(g) 2(g)) on the left side, there is more "stuff" increased in the ...
Fundamentals of Combustion
... 1.2.1 Thermal explosion theory . . . . . . . . . . . . . . . . . . 1.2.1.1 One-step reversible kinetics . . . . . . . . . . . . 1.2.1.2 First law of thermodynamics . . . . . . . . . . . 1.2.1.3 Dimensionless form . . . . . . . . . . . . . . . . . 1.2.1.4 Example calculation . . . . . . . . . . . . . ...
... 1.2.1 Thermal explosion theory . . . . . . . . . . . . . . . . . . 1.2.1.1 One-step reversible kinetics . . . . . . . . . . . . 1.2.1.2 First law of thermodynamics . . . . . . . . . . . 1.2.1.3 Dimensionless form . . . . . . . . . . . . . . . . . 1.2.1.4 Example calculation . . . . . . . . . . . . . ...
Forward
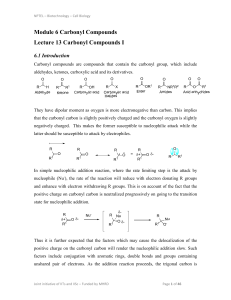
... As we’ll see later in this chapter and the next, aldehydes and ketones are involved in many of the most used reactions in synthetic organic chemistry. Where do aldehydes and ketones come from? Many occur naturally. In terms of both variety and quantity, aldehydes and ketones rank among the most comm ...
... As we’ll see later in this chapter and the next, aldehydes and ketones are involved in many of the most used reactions in synthetic organic chemistry. Where do aldehydes and ketones come from? Many occur naturally. In terms of both variety and quantity, aldehydes and ketones rank among the most comm ...
ADSORPTION OF Cr(NH3)6 3+ AND Cr(en)3 3+ ON CLAY
... than for the Cr(NH3)63+ complex. For kaolinite the chromium adsorbed is almost doubled, from 5 ppm for the Cr(NH3)G3§ complex to 9 ppm for the Cr(en)33+ complex. These results are similar to those previously reported (Koppelman and Dillard, 1977) for the adsorption of other transition metal ions on ...
... than for the Cr(NH3)63+ complex. For kaolinite the chromium adsorbed is almost doubled, from 5 ppm for the Cr(NH3)G3§ complex to 9 ppm for the Cr(en)33+ complex. These results are similar to those previously reported (Koppelman and Dillard, 1977) for the adsorption of other transition metal ions on ...
Chapter 6 Thermodynamics: The First Law
... Describe the state of the following system: a partition that separates a sample of liquid water at 10°C from a sample of water at 30°C is removed. ...
... Describe the state of the following system: a partition that separates a sample of liquid water at 10°C from a sample of water at 30°C is removed. ...
Study materials of Chemistry for class XII
... Q10.Noble gases and metals crystallise with closed packed structures, yet the meeting points of noble gas crystals are exceptionally low. Why? 1M Ans. Noble gases crystallise in close packed structures, but the forces of interaction between the atoms are weak dispersion forces and they therefore hav ...
... Q10.Noble gases and metals crystallise with closed packed structures, yet the meeting points of noble gas crystals are exceptionally low. Why? 1M Ans. Noble gases crystallise in close packed structures, but the forces of interaction between the atoms are weak dispersion forces and they therefore hav ...
NAME - HCC Learning Web
... The conjugate acid to (CH3)3N: is (CH3)3NH+. An electron pair donor substance considers being a Lewis acid. The stronger the acid, the weaker the conjugate base. ...
... The conjugate acid to (CH3)3N: is (CH3)3NH+. An electron pair donor substance considers being a Lewis acid. The stronger the acid, the weaker the conjugate base. ...
Chapter 14 - Chemistry Solutions
... Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. ...
... Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. ...
Ethers, Epoxides, and Sulfides
... Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. ...
... Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. ...
Chemistry - A Quantitative Science
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
Electrochemical and Spectroelectrochemical Studies of Dyes used
... could be generated by light studying the interaction between light and matter was Edmond Becquerel who discovered the photovoltaic effect in 18391 while experimenting with an electrolytic cell made up of two metal electrodes placed in conducting solution. He observed that current increased when the ...
... could be generated by light studying the interaction between light and matter was Edmond Becquerel who discovered the photovoltaic effect in 18391 while experimenting with an electrolytic cell made up of two metal electrodes placed in conducting solution. He observed that current increased when the ...
coordination compounds
... oxygen transport to the tissues of the body. Permanent exchange of substances to the environment enables to the body maintain a certain level of concentration of the compounds involved in the equilibrium of the complexation processes, providing metal-ligand homeostasis. In addition, complex compound ...
... oxygen transport to the tissues of the body. Permanent exchange of substances to the environment enables to the body maintain a certain level of concentration of the compounds involved in the equilibrium of the complexation processes, providing metal-ligand homeostasis. In addition, complex compound ...
Alkyl Chain Length Dependence of the Dynamics and Structure in
... small but measurable peak shift and a slight broadening as the alkyl chain length is increased. The changes in the spectra, though minor, are an indication that the chain length does have some impact on the structure of the ionic regions, resulting in changes in the intermolecular interactions betwe ...
... small but measurable peak shift and a slight broadening as the alkyl chain length is increased. The changes in the spectra, though minor, are an indication that the chain length does have some impact on the structure of the ionic regions, resulting in changes in the intermolecular interactions betwe ...