06. Alcohols. Phenols. Ethers
... Тhe alkyl alcohol system. In this system of common nomenclature, the name of an alcohol is derived by combining the name of the alkyl group with the word alcohol. The names are mitten as two words. ...
... Тhe alkyl alcohol system. In this system of common nomenclature, the name of an alcohol is derived by combining the name of the alkyl group with the word alcohol. The names are mitten as two words. ...
Ch 18 Power Point
... • Catalysts have no effect on relative equilibrium amounts. • They only affect the rates at which equilibrium is reached. • Catalysts increase the rates of forward and reverse reactions in a system by equal factors. Therefore, they do not affect K. ...
... • Catalysts have no effect on relative equilibrium amounts. • They only affect the rates at which equilibrium is reached. • Catalysts increase the rates of forward and reverse reactions in a system by equal factors. Therefore, they do not affect K. ...
CH 3
... 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
... 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
CH 3
... 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
... 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
Ch22 Test
... 39. (Petroleum, Natural gas) is a mixture of alkanes, aromatic hydrocarbons, and organic compounds containing sulfur or nitrogen atoms. ____________________ 40. The boiling of petroleum and collection of its components is called (sedimentation, fractional distillation). ____________________ 41. In t ...
... 39. (Petroleum, Natural gas) is a mixture of alkanes, aromatic hydrocarbons, and organic compounds containing sulfur or nitrogen atoms. ____________________ 40. The boiling of petroleum and collection of its components is called (sedimentation, fractional distillation). ____________________ 41. In t ...
The Chemistry and Applications of Metal
... Design of Ultrahigh Porosity During the past century, extensive work was done on crystalline extended structures in which metal ions are joined by organic linkers containing Lewis base–binding atoms such as nitriles and bipyridines (8, 9). Although these are extended crystal structures and not large ...
... Design of Ultrahigh Porosity During the past century, extensive work was done on crystalline extended structures in which metal ions are joined by organic linkers containing Lewis base–binding atoms such as nitriles and bipyridines (8, 9). Although these are extended crystal structures and not large ...
Chemistry - SSA Punjab
... of 1.83g mL-1 (i) Calculate the molarity of the solution (ii) volume of concentrated acid required to prepare 3.5L of 0.50 M H2SO4 ...
... of 1.83g mL-1 (i) Calculate the molarity of the solution (ii) volume of concentrated acid required to prepare 3.5L of 0.50 M H2SO4 ...
deltahpps
... Calculate standard enthalpy changes using bond enthalpy values Calculate standard enthalpy changes using enthalpies of formation and combustion Know simple calorimetry methods for measuring enthalpy changes Calculate enthalpy changes from calorimetry measurements ...
... Calculate standard enthalpy changes using bond enthalpy values Calculate standard enthalpy changes using enthalpies of formation and combustion Know simple calorimetry methods for measuring enthalpy changes Calculate enthalpy changes from calorimetry measurements ...
Rice Chemistry Advising Booklet - Rice University Department of
... CHEM 212 is primarily taught as a large lecture course much like CHEM 211. CHEM 320 is a small course — 12 to 25 students — and is intended for students considering chemistry as a major and those in related fields with a strong interest in chemical research. Taught in small groups, CHEM 320 minimize ...
... CHEM 212 is primarily taught as a large lecture course much like CHEM 211. CHEM 320 is a small course — 12 to 25 students — and is intended for students considering chemistry as a major and those in related fields with a strong interest in chemical research. Taught in small groups, CHEM 320 minimize ...
Visible Light Photoredox Catalysis with Transition
... may be transiently generated in the same reaction vessel, photoredox approaches may be used to develop reactions requiring both the donation and the reception of electrons at disparate points in the reaction mechanism. This approach stands in contrast to methods requiring stoichiometric chemical oxi ...
... may be transiently generated in the same reaction vessel, photoredox approaches may be used to develop reactions requiring both the donation and the reception of electrons at disparate points in the reaction mechanism. This approach stands in contrast to methods requiring stoichiometric chemical oxi ...
Mole-mole factor
... A balanced chemical equations tell us: – The formulas and symbols of the reactants and products – The physical state of each substance – If special conditions such as heat are required – The number of molecules, formula units, or atoms of each type of molecule involved in the reaction • Number can b ...
... A balanced chemical equations tell us: – The formulas and symbols of the reactants and products – The physical state of each substance – If special conditions such as heat are required – The number of molecules, formula units, or atoms of each type of molecule involved in the reaction • Number can b ...
Ans:- (i) Gluconic acid - Kendriya Vidyalaya No.2, Kribhco, Surat
... Q-7 How does an electrochemical cell help in predicting the feasibility of a redox reaction ? Ans-14.If E0 of the cell is +ve it will yield –ve ∆G0 Value which indicates the reaction is spontaneous. ∆G0 = -n F E0 Q.8 Why m for acetic acid cannot be determined experimentally? Ans. Molar conductiv ...
... Q-7 How does an electrochemical cell help in predicting the feasibility of a redox reaction ? Ans-14.If E0 of the cell is +ve it will yield –ve ∆G0 Value which indicates the reaction is spontaneous. ∆G0 = -n F E0 Q.8 Why m for acetic acid cannot be determined experimentally? Ans. Molar conductiv ...
technical report 91 -32
... It is generally accepted that reducing conditions exist in the far field of a repository, because the rocks contain a number of reducing minerals. They can reduce adsorbed radionuclides directly and/or they are dissolved and reduce the radionuclides in solution. For this reason, the redox conditions ...
... It is generally accepted that reducing conditions exist in the far field of a repository, because the rocks contain a number of reducing minerals. They can reduce adsorbed radionuclides directly and/or they are dissolved and reduce the radionuclides in solution. For this reason, the redox conditions ...
Stoichiometric Calculations
... This formula can be read as:* 1 L of CS2 + 3 L of O2 yields 1 L of CO2 + 2 L of SO2 This approach only works when comparing two gases in a formula. It cannot be used to compare a gas to a liquid or solid. [ * at STP conditions: 1 x 22.4 L + 3 x 22.4 L → 1 x 22.4 L + 2 x 22.4 L ...
... This formula can be read as:* 1 L of CS2 + 3 L of O2 yields 1 L of CO2 + 2 L of SO2 This approach only works when comparing two gases in a formula. It cannot be used to compare a gas to a liquid or solid. [ * at STP conditions: 1 x 22.4 L + 3 x 22.4 L → 1 x 22.4 L + 2 x 22.4 L ...
Iridium(III) and Rhodium(III) compounds of dipyridyl-N
... acetonitrile showed fragmentation of molecular ion peaks at m/z = 470 and 435 due to ([(η5 -C5 Me5 )Rh {(C5 H4 N)2 C=N-Me}Cl])+ ([2]PF6 -PF6 )+ and ([(η5 C5 Me5 )Rh{(C5 H4 N)2 C=N-Me}])+ ([2]PF6 -PF6 -Cl)+ (see Supplementary Information). In addition, the spectrum also showed peak at m/z 421 due to ...
... acetonitrile showed fragmentation of molecular ion peaks at m/z = 470 and 435 due to ([(η5 -C5 Me5 )Rh {(C5 H4 N)2 C=N-Me}Cl])+ ([2]PF6 -PF6 )+ and ([(η5 C5 Me5 )Rh{(C5 H4 N)2 C=N-Me}])+ ([2]PF6 -PF6 -Cl)+ (see Supplementary Information). In addition, the spectrum also showed peak at m/z 421 due to ...
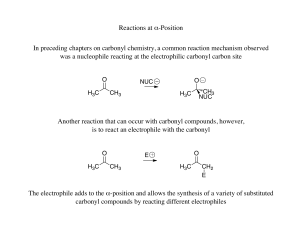
Organic Chemistry II Introduction
... • The one -step mechanisms is E2 reaction and two step reaction mechanism is called E1 reaction, respectively. Dr Seemal Jelani ...
... • The one -step mechanisms is E2 reaction and two step reaction mechanism is called E1 reaction, respectively. Dr Seemal Jelani ...
Hydrogenation, Transfer Hydrogenat- ion and Hydrogen Transfer Reactions
... triggers and participates in the reaction to make the reaction go faster by decreasing the activation energy, without itself being consumed. It does not change the equilibrium of reaction system, but accelerates the reaction by stabilizing the transition state. As it is not included in the product, ...
... triggers and participates in the reaction to make the reaction go faster by decreasing the activation energy, without itself being consumed. It does not change the equilibrium of reaction system, but accelerates the reaction by stabilizing the transition state. As it is not included in the product, ...
Mock Exam One
... a.) The bond angle around the oxygen of an alcohol is < 109.5°. b.) Alcohols contain a polar covalent bond that an alkane does not have. c.) The strongest intermolecular force present in an alcohol is hydrogen bonding while the strongest intermolecular force present in alkanes is London dispersion. ...
... a.) The bond angle around the oxygen of an alcohol is < 109.5°. b.) Alcohols contain a polar covalent bond that an alkane does not have. c.) The strongest intermolecular force present in an alcohol is hydrogen bonding while the strongest intermolecular force present in alkanes is London dispersion. ...