* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Kinetic Assay of Human Pepsin with Albumin
Thermomechanical analysis wikipedia , lookup
Kinetic resolution wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Western blot wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Biochemistry wikipedia , lookup
Catalytic triad wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Analytical chemistry wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Virus quantification wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Transition state theory wikipedia , lookup
Biosynthesis wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
CLIN. CHEM. 29/3, 447-451 (1983)
Kinetic Assay of Human Pepsin with Albumin-Bromphenol
Substrate
Blue as
Stephen P. Gray and John A. Billings
A novelsubstrate,albumincomplexedwith bromphenolblue,
has been developed for the assay of human gastric juice
pepsin by a kinetic method in the Cobas centrifugal analyzer.
The action of pepsin on the complex degrades the albumin
and releases the dye. The change in the color of the
substrate is a zero-order reaction. Human and porcine pepsin have different Km’Swith the new substrate. This kinetic
methodhas a throughputof 28 tests in approximately10 mm
and good precision (CV = 2.0%). Other advantages are
analysis in homogeneous solution (thereby eliminating the
need to separate substrate and products), lack of interfer-
ence from bilirubin or phenol red, and the expression of
pepsin activity in IUB enzyme units.
AdditionalKeyphrases:gastric juice
sis
centrifugal analydifferences between human and porcine pepsin
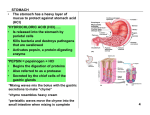
Pepsins (EC 3.4.23.1) are secreted in gastric juice as
inactive pepsinogens,
of which eight are known (1). The
pepsinogens are cleaved at pH 5 to form the actiye enzyme.
Once cleavage has begun, it proceeds autocatalytically
in
the hydrochloric acid normally present in the stomach. The
main source of pepsin is the chief cell located in small pits in
the gastric mucosa. In the stomach, pepsins appear to act
primarily
on peptide bonds in the middle of the protein
molecule, which are made accessible by hydrochloric
acid
denaturation;
thus, the active pepsins
are endopeptidases
and show the greatest activity against aromatic amino acids
such as tryptophan,
phenylalanine,
and tyrosine (2).
The assay of pepsin depends on estimating the number of
peptide bonds broken during the rcaction with a protein
substrate. Some authors have used dried plasma proteins (3)
or edestin (4) as a substrate,
but bovine hemoglobin and
albumin
have been the most widely used. In general the
substrate is incubated
at pH 2.0 with the pepsin (optimal pH
range 1.8-3.5) and the liberated
tyrosine is then reacted
with Folin phenol reagent (5) or the absorbance
is read at
275-280 tim (6). Most such methods compare the enzyme
activity vs that of a reference
preparation
of hog mucosal
pepsin, which differs in potency according to the degree of
purification,
and the units of pepsin activity are then
reported in terms of “tyrosine equivalents,”
“hog pepsin
units.” or “absorbance units” (6).
Use of protein substrates suffers from the technical drawback that the released soluble products of pepsin digestion
have the same optical and chemical properties as the intact
amino acids in the protein chain and therefore have to be
physically separated
from the original substrate, usually by
centrifugation,
ifitration, or dialysis, before the change in
absorbance
can be measured.
Such procedures
militate
against the development of kinetic methods of analysis for
the enzyme, which require homogeneous
solution chemistry. Furthermore,
the different expressions for the units of
enzyme activity prevent accurate
comparisons with results
from other investigative
centers.
In general,
research
groups have tended to establish their own reference ranges
for the given experiment,
with the result that the reported
values often do not have universal applicability.
Moreover,
no pure, stable human pepsin is yet available as a reference
standard. Most enzymes are measured kinetically in clinical
chemical laboratories,
which also takes advantage
of the
various automated
analyzers devised specifically for that
purpose. The need for a new method of measuring
human
gastric
pepsin by reaction-rate analysis in homogeneous
solution,
such that the activity could be expressed in standardized units in common with most other enzymes, is
undeniable. The present study attempts to fulfill it.
Materials and Methods
Human
gastric juice pepsin having its optimal activity
around pH 2.0 (2), we decided to measure at that pH, using
glycine HC1 buffer (0.4 mol/L) to maintain it.
Buffer. Glycine HC1, 0.4 molJL, pH 2.0 at 37 “C; reagentgrade glycine was obtained
from BDH, Poole, Dorset, U.K.
Albumin.
Bovine albumin was purchased as “Albumin
Stock Solution,”
10 g/100 mL, code no. 905-10 (Sigma
Chemical Co., St. Louis, MO 63178).
Bromphenol
blue. Purchased
as a thy powder, “pH Indicator” (Hopkin and Williams, Chadwell Heath, Essex, U.K.).
Dissolve the appropriate amount of dye (Mr 670.02) in the
minimum amount of ethanol, dilute to the mark with pH 2.0
glycine buffer, and recheck the pH. The stock solution was
1.0mmol/L.
Substrate.
For use in the automatic analyzer, add 1.80 mL
of the Albumin Stock Solution to 3.0 mL of the bromphenol
blue stock solution, mix well, and make up to 20 mL with
pH 2.0 glycine buffer; re-mix. This reagent can be placed in
the reagent tray ready for use. Its final concentration in the
cuvette is 150 prnol/L.
Samples. Gastric juice, ifitered or centrifuged before analysis.
Procedures
Substrate
development.
Albumin combines
with bromphenol blue at pH 2.0 to give a product having a different
color from the original dye. Pepsin acting on this complex
appears to break it up and regenerate
the free bromphenol
blue. These color changes can be followed over time.
Composition
of the albumin-bromphenol
blue complex.
We determined the composition of the complex by application of the law of mass action to the assumed equilibrium:
k
of Biochemistry, Royal Naval Hospital,
Gosport, Hampshire P012 2AA, U.K.
Received Oct. 15, 1982; accepted Dec. 14, 1982.
Department
Haslar,
niALB
+ nBPB
where k is the stability
ALBm
constant,
-
BPB
ALI3 represents
albumin,
CLINICALCHEMISTRY,Vol.29, No. 3, 1983 447
BPB is bromphenol blue, and m and n are the numbers
molecules
reacting. Rearranging,
k
=
of
raphy on activated
[ALBmBPBnI
[ALB]m- [BPB]”
from the double-log
plot provide
an
estimate of the values fcr m and n, thereby giving the molar
composition of the complex. To calculate the stability constant, k, we substituted appropriate pairs of related data
into the rearranged
expression shown above.
Spectrophotometry.
The spectral chatiges of the new complex with time in the presence of human gastric juice pepsin
were examined
with a Cary 219 recording spectrophotometsr, in an attempt to determine the optimum wavelength for
studying the reaction.
Kinetics. Curves relating absorbance
change with the new
substrate
to differing pepsin concentrations were plotted to
calculate Km and V,
and hence the optimal
substrate
concentration. Conditions for zero-order
rate reaction were
established
by studying the time course of the pepsinsubstrate reaction. Km and V,
were calculated by using
the Hanes plot (7). We also examined the effect of temperature on the reaction and constructed
an Arrhenius
plot of
the data relating temperature
to pepsin activity, from which
we calculated the activation energy and the temperature
coefficient, Qio, the factor by which the rate increases when
the temperature
is increased by 10#{176}C.
Auto,nated
analysis.
We adapted the manual spectrophotometer assay for pepsin to the Cobas Bio Centrifugal
Analyzer (Roche Ltd., Welwyn Garden City, U.K.) because
we found that the period of zero-order kinetics was difficult
to determine manually. The Cobas, however, had the facility to detect the linear portion of the reaction and then
calculate the best fit by a least-squares regression analysis.
Use of this instrument
allowed us to express the results in
terms of IUB enzyme units (U) of activity at 37#{176}C
and to
automate
the whole assay for pepsin.
Correlation
with a reference method of pepsin assay. We
used the method of Burstad (6), which correlates very closely
with the earlier, classical method of Anson (5). The latter is
used in the United States, whereas the Burstad method is
popular in Europe.
We analyzed 92 samples of human
gastric juice obtained post-stimulation
with pentagastrin
and insulin and calculated the correlation.
The Burstad
method in outline is as follows. Add diluted gastric juice to
human hemoglobin substrate in HC1 at pH 2.0 and incubate
at 25 #{176}C
for 10 min. Then, to the mixture add trichloroacetic
acid to precipitate the unchanged substrate proteins. Filter
these off and read the absorbance
of the ifitrate at 280 rim,
expressing the pepsin activity in absorbance
units, or compare the results with those for a reference porcine pepsin
preparation treated in the same way as the gastric juice
samples.
Action of pepsin on the new substrate.
The action of pepsin
on albumin is well known (2), but we had to test the
possibility
that complexation with bromphenol blue may
alter the chemistry
of the reaction. To test this thesis,
porcine pepsin (Boehringer
Mannheim,
BCL, Lewes, Sussex, U.K.) and human gastric pepsin were allowed to react
448
CLINICALCHEMISTRY,Vol.29, No.3, 1983
silica gel (8).
between IUB units and Anson
units. Although many pepsin units are in current use, we thought we
Relationship
We set up two experiments,
in one keeping the albumin
concentration constant and varying that of the bromphenol
blue, the second keeping the bromphenol blue constant and
varying the albumin. The absorbances of all the solutions
were measured at 605 nm and the values of m and n were
evaluated by plotting the logarithms of absorbances against
the logarithms
of the molar concentrations of each reactant.
The slopes of the curves
with albumin
alone and with albumin-bromphenol
blue at
pH 2.0 in 0.4 mol/L glycine HC1 buffer until apparent
equilibrium
was attained.
The products of digestion were
then analyzed for free amino acids by thin-layer chromatog-
could establish the relationship between IUB enzyme units
(1 U = 1 mol of substrate converted per minute) and Anson
units (5), in which the activity of commercial porcine
preparations
is commonly expressed.
One Anson unit is the
enzyme activity that liberates under specified assay conditions sufficient tyrosine to increase
the absorbance
at 280
rim by 0.001/ruin. We tested known amounts of porcine
pepsin with the new assay system
and calculated
the
equivalent
in IUB enzyme units.
Possible interferences.
Bilirubin,
not normally
present
in
gastric juice, may occasionally appear as a consequence of
duodenal refiux. To check whether it interfered with the
new assay, we obtained specimens
of gastric juice heavily
contaminated
with bilirubin from such reflux, mixed them
with samples of clear, uncontaminated
gastric juice, and reanalyzed the resulting mixtures.
Phenol red, a “nonabsorbable
ion” given by constant
infusion via the stomach tube to provide an estimate-by
calculating
its recovery-of
the extent of gastric gains and
losses by reflux, was tested as a possible interferent
by
addition in various proportions (from zero to 100% dye) to
the gastric juice and re-analyzing.
Statistical methods. Linear-regression
analysis, mean and
SD, and Student’s t-test were taken from Colquhoun
(9). For
all calculations
we used an HP-9815 desk-top calculator and
the statistical programs
provided by the manufacturer
(Hewlett Packard Ltd., Wokingham,
U.K.).
Protocol for pepsin assay on the Cobas Bio. In principle,
substrate is pipeted by the instrument
into the rotor cuvette
equilibrated
to 37 “C; gastric juice (10-20 tL) is then added
and mixed. The first reading is taken 0.5 s after this mixing
and sibsequent
readings made at 10-s intervals
for 5 min.
The Cobas measures the absorbance
change per minute,
searches for the start of a linear path of decreasing absorbance at 605 nm, and calculates
a least-squares regression
analysis
on the maximum number of points lying within a
bandwidth of ±0.0025 absorbance
units (A).
A typical format for the analysis is as follows:
1. Units
U/L
2. Calculation factor
1041
(incorporates
molar absorptivity)
3. Temperature,
#{176}C 37.0
4.
Type of analysis
5.
Wavelength,
6.
Sample volume,
7.
Diluent
volume,
8.
Reagent
volume,
9.
Incubation
time, s
Time of first read-
2
tim
(kinetic,
decreasing
sorbance)
ab-
605
10.
ing,
11.
12.
13.
S
Time
interval,s
No. of readings
20
(gastric
juice)
20
(distilled water)
200
(substrate)
180
(before sample addition)
0.5
10
30
1
(blanks after all added
to cuvette)
The centrifugal
rotor holds 28 samples of gastric juice,
which are analyzed concurrently.
The incubation interval
before analysis is 3 mm and the analysis time is 5 mm;
Blanking
mode
I.--.
I
04
.
-----4------
-0.--------
04
05
-
DI
10gb
/
-
-
-
I
1*
4
i.
1I
UiS, hauL
n,JI....l
Fig. 1. Logarithmic plot to determine binding parameters for the
albumin-bromphenol blue reaction
The slope of this double-icy plot equates to the numericalvalue of the molar
ratios,m and n, inthe equationfor k (see text for details)
therefore, the time taken
for the 28 tests to be completed is
about 10 mm.
Results
Composition
of the albumin-bromphenol
blue
complex.
Using the logarithmic
method described
in Methods,
we
determined
that albumin and bromphenol
blue react in a
one-to-one molar ratio. The value of k, the stability constant,
was 3.72 x i0, which indicates that very little free dye or
albumin is present when the two compounds are allowed to
react in unimolar
proportions. One of the curves is illustrated in Figure 1.
Spectral properties of the complex. In Figure 2 the spectral
scans of the free bromphenol blue and the complex with
albumin are illustrated superimposed to show the change in
absorption. There are two absorption peaks, at 450 nm and
605 tim; we chose to monitor the one at 605 nm for the
reaction with pepsin because the absorbance
values there
are much lower and thus in the range of most spectrophotometers. Using this wavelength
meant that a decrease in
absorbance with time was monitored.
When the enzyme
reaction
was allowed
to go to equilibrium,
the spectral
absorbance of the products was indistinguishable
from that
of the uncomplexed dye.
Kinetics of the reaction. The rate of change of absorbance
per minute was plotted against a series of different substrate
concentrations reacted with a high activity sample of human gastric juice. The resulting data wlore plotted according
to the Hanes procedure (7). The results of 12.0 prnolJL for
:Ji
.-“
I,
/.
F
I
/
/
r
,/
/
‘
\
/
I
I.,
I
I
I
,
s___,
0.50
‘
00
400
450
500
560
600
,eo
nm
Fig. 2. The change in absorbance at 605 nm when the substrate,
albumin-bromphenol blue (curve 2), is aftacked by pepsin at pH 2.0 to
yield the free bromphenol blue (curve 1)
p710
-Km
0
(ANumIn-Brompheeol
Blus)[S),p mol/L
Fig. 3. Effect of varying the substrate(albumin-bromphenolblue)
concentration [S] in the presence of a constant amount of human
pepn
y.axis, LSVA substrateooncentration/absorbance
=
and of 0.341 mol/min
for V
were checked by means
of an iterative calculation for each (7) and both techniques
were in agreement.
An illustrative Hanes plot is shown in
Figure 3. To ensure saturation
of the enzyme, we used a
final substrate concentration of approximately 10-fold the
Km value, which gave a zero-order
rate reaction.
The
absorbance
value of this concentration
of substrate
was
approximately
1.2 A, which is well in the range of most
enzyme analyzers. The molar absorptivity for the complex
was 12 x io L mol
cm1 at 605 rim.
Data obtained with the Cobas analyzer, which can read
the absorbance
every 10 s until the time to equilibrium,
indicated that the time needed to reach zero-order rate was
a function of the pepsin concentration.
It was necessary
to
follow each test for 5 rain to accumulate
sufficient data
points for a least-squares
regression
analysis
within the
close limits set by the Cobas analyzer. Specimens of human
gastric juice with low pepsin activity showed a “lag” phase of
up to 1 mm before the linear path commenced; in samples of
high activity this lag phase lasted only a few seconds.
Effect of temperature
on the reaction. We used a specimen
of human gastric juice with high peptic activity to study the
effect of increasing
temperature
on the rate of the reaction.
The plot of the data is shown in Figure 4 as an Arrhenius
diagram. The calculated activation energy was 74.88 kJ/
mol, and the temperature
coefficient, Qio, was 2.5, indicating that the rate of the reaction was multiplied by that
amount for each 10#{176}C
rise in temperature.
There was no
evidence of a transition point in the range of temperatures
studied.
Correlation
with a comparison
method. We used the
method of Burstad (6) as a reference
for pepsin
assay.
Samples of gastric juice aspirated
after stimulation
with
pentagastrin
and insulin were assayed for pepsin activity by
the new method and by the method of Burstad. The resultKm
-
CLINICALCHEMISTRY, Vol. 29, No. 3, 1983 449
4.
50
44
42
4
3
6
Activation
Qio
‘.
-
-
-
-
-
-
-
e#{176}C pepsin had an activity
4
74
Energy
kJ/mol
2.5.
-
-
-
-
-
-
-
-
-
-.
-
“c;;
-
R20.982
-
-
-
-
-
-
N
4.50
-
4.00
-
310
-
-
312
-
-
314
-
-
3*
-
315
-
-
-
-
320
-
-
-
-
-
-
5
324
-
-
330
5 2
1/OK (x105)
Fig. 4. Arrhenius plot of the effect of changing the temperatureat which
human pepsin reacts with albumin-bromphenol blue
The high value for Q,, indicatesthat the enzymes specific activity increases
rapidly with increasing temperature
of 2500 Anson units (5) per milligram; therefore, 16 pg would have a specific activity of
approximately
40 Anson units. Thus the approximate
relationship between units was: 1 Anson unit
10 UIL.
Kinetics
of porcine pepsin. The same porcine pepsin was
investigated for its kinetic properties in the new substrate.
We found that its Km was 80 prnol/L, i.e., six- to sevenfold
the value for human pepsin found in the gastric juice from
healthy people.
Within-run analysis. We re-analyzed gastric juice samples
and subjected the data to linear-regression analysis and the
paired t-test. In a typical set of results (n = 15) we found the
following: means 124.01 and 124.11 UIL and the t-test for
paired values 0.1 (CV = 2.0%). The range of values tested
was 20.29-387.28
UIL (SD 94.8 UIL).
Between-run
analysis.
We carried out these analyses on
fresh samples. The CV was 1.86%, and the mean differences
were statistically insignificant.
Stability
studies.
We investigated
the effect on pepsin
stability of storage in the frozen state. We analyzed 20
samples with values ranging from 3.3 to 181.0 U/L before
and after storage at -20 #{176}C.
The mean value before storage
was 95.6 UIL and after 93.8 UIL. The t-value was significant
at the 5% level and we concluded there was a change in
pepsin recovery after freezing and storage at this temperature. On the other hand, stability studies on pepsin in
gastric juice stored at -85 #{176}C
indicated
no significant deterioration at that temperature.
Interference from additional substances. The commonest
contaminant
of gastric juice is bile derived from duodenal
refiux, so we added duodenal juice to gastric juice and reassayed the samples for pepsin by the new method. We
analyzed 20 samples
and found no significant difference
between the original values and those after addition of bile
(t
240am
Fig. 5. Correlation between the new automated kinetic method with
albumin-brornphenol blue substrate (y) and the manual method of
Burstad (6) (Jc)
Thesetwo very differentmethodsagreeclosely(
0.984) over a wide range of
enzymeactivities.The regressionequationis: pepsin, U/L -4.41 + 251.3 A (at
280 nm). SD of the x-varlable 0.43 (mean 0.62) and SD of the y.varlable =
108.0(mean 151.6)
=
=
ing data for 92 specimens are illustrated
in Figure 5. The
correlation was good, r2 = 0.984.
Both methods gave identical post-stimulation
patterns and appear to-be measuring
the same thing.
Effect of pepsin on the substrate.
To check that complexing
bromphenol blue with albumin did not alter fundamentally
the chemistry of the action of pepsin on the albumin part of
the complex, we used thin-layer chromatography in butanoll
acetic acid/water (60/30/10, by vol) to separate the products
after digestion. The amino acid pattern from the albumindye complex was indistinguishable
from that for uncomplexed albumin. We concluded, therefore, that not only did
the dye not materially affect the protein digestion by pepsin
but also the results were compatible with the idea that the
enzyme attacked the albumin to release amino acids and
detached the dye from the remnant
protein.
Correlation
between enzyme units and Anson units.”Porcine pepsin, prepared from crystalline
pepsin and lyophilized, was studied in the new assay system in the same way
as human gastric juice pepsin. Analysis of 10 samples of 20
1L, each containing
16 &g of porcine pepsin, gave the
following results: mean 418.5 UIL (at 37#{176}C),
SD 8.6 U/L,
and CV 2.0%. According
to the manufacturer,
the porcine
450
CLINICAL CHEMISTRY, Vol. 29, No. 3, 1983
=
0.53).
Another contaminant
of gastric juice in experimental
work is phenol red, used to determine reflux losses, and we
used a similar procedure to the above, adding the dye in
several proportions
to the gastric juice. The mean value
before addition was 137.0 UIL and after the mean was 138.6
UIL (t = 0.92). We concluded that phenol red does not
interfere with the assay.
Normal values. The normal range for pepsin cannot be
established
in the usual way because the enzyme is secreted
in response to many physiological and psychological factors.
We report basal (i.e., nonstiniulated)
values for pepsin,
which we found to be about 2.5-10.2
U/h (mean ±2 SD).
Discussion
The chemistry of the reaction of albumin-bromphenol
blue with pepsin is not simple. Albumin combines with the
dye at pH 2.0 to give a dark red compound that has a slight
green fluorescence. During attack by the enzyme the color
changes to give the yellow green of the free bromphenol blue
and the fluorescence disappears.
The complexed dye does
not appear to alter the types or amounts of amino acids
released from albumin by digestion with the pepsin, so we
assume that the protein part of the substrate is degraded in
the usual manner (2) and that the previously bound dye is
then detached from the digested remnants.
If the rate of
release of the dye is a function of the enzyme activity, as it
appears to be, then we can use it as an assay for pepsin. By
regulating the conditions we can obtain zero-order kinetics
and, consequently,
can express the activity in IUB enzyme
units.
It is possible that under the same conditions
tyrosine
release may be zero-order and could be measured kinetically, but the drawback is that tyrosine in the intact albumin
substrate has similar physical and chemical properties to
the free amino acid in the product matrix and it is necessary
to separate, by physical means, the substrate and products.
This is the procedure adopted in the classical methods for
pepsin assay (5,6,10).
The proposed kinetic method has the
advantage
of being carried out in homogeneous solution.
Use of several systems of pepsin units precludes useful
comparison
of results from different centers; expressing
pepsin activity in the same JUB enzyme units as are most
other enzymes of clinical interest should facilitate comparisons of experimental
and clinical findings. It is a widespread
practice to use porcine pepsin as a reference standard in the
absence of the availability of a pure human pepsin preparation, but kinetic methods of analysis do not require an
external reference. Furthermore,
we noted differences
in
behavior between porcine pepsin and human gastric juice
pepsin, so that, in this system at least, porcine pepsin would
not be a satisfactory
standard. Clearly, human pepsin, when
available,
may be used to calibrate
the present kinetic
method. The converse is true also: this method may be used
to compare the potency or degree of activity of any given
pepsin preparation.
Absence of interference
from products derived from duodenal refiux or deliberately added substances such as phenol
red is an important asset and, once the optimum is established, the analysis
appears to proceed despite their presence.
Standardization
of pepsin assays in the manner we propose should allow large amounts of data to be collected and
subjected to statistical
analysis, which should illuminate
this area of gastric pathophysiology.
Pepsin dynamics are
still poorly understood
after decades of investigation (11)
and this new method may help in their clarification.
We acknowledge the help and provision of samples of human
gastric juice from the Department of Experimental Gastroenterology, Royal Naval Hospital, Haslar, Gosport, U.K. Especial thanks
are due to Professor R. H. Hunt, FRCP, Professor of Medicine at
McMaster University Medical Center, Hamilton, Ontario, Canada,
and Mr. T. Gledhill, FRCS, in the Division of Gastroenterology,
McMaster, for valuable clinical discussions during the development
of this paper.
References
1. Samloff IM. Slow moving protease and the seven pepsinogens.
57, 659-669 (1969).
2. Taylor WH. Biochemistry of pepsin. In Handbook of Physiology,
5, Sect. 6, Alimentary Canal, CF Code, Ed., American Physiological
Gastroenterology
Soc., Washington,
DC, 1968, pp 2567-2587.
3. Hunt JN. A new method for estimating peptic activity in gastric
contents. Biochem J 42, 104-109(1948).
4. Wynn-Williams A. A simple method for the estimation of pepsin
in gastric juice. J Clin Pat/wi 8,85 (1955).
5. Anson ML. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J Gen Physiol 22, 79-89 (1938).
6. Burstad A. A modified hemoglobin substrate method for the
estimation of pepsin in gastric juice. Scand J Gastroenterol 5,343348 (1970).
7. Cornish-Bowden A. Principles of Enzyme Kinetics, Butterworths,
London, 1976, pp 25-27.
8. Ireland JT, Read RA. A thin layer chromatographic method for
use in neonatal screening to detect excess amino acidaemia. Ann
Clin Biochem 9, 129-132 (1972).
9. Colquhoun D. Lectures in Biostatistics, Clarendon Press, Oxford,
London, 1971.
10. Klotz AP, Duvall MR. The laboratory determination of pepsin
in gastric juice with radioactive-iodinated albumin. JLab Clin Med
50, 753-757
(1957).
11. Achord JL. Gastric pepsin and acid secretion in patients with
acute and healed duodenal ulcer. Gastroenterology 81,15-18(1981).
CLINICALCHEMISTRY, Vol. 29, No. 3, 1983 451