Other High Energy Compounds
... stores are high. Ingestion of creatine has been proven to help in performance in high intensity short term exercise. Creatine slows the fatigue process because during the rest stage there is larger amount of ATP replinshed compared to a person who is not taking creatine. ...
... stores are high. Ingestion of creatine has been proven to help in performance in high intensity short term exercise. Creatine slows the fatigue process because during the rest stage there is larger amount of ATP replinshed compared to a person who is not taking creatine. ...
Cell Respiration and Fermentation PPT
... and reproduce, maintain their structures, and respond to their environments. – Usually divided into two categories. Catabolism and Anabolism − Catabolism: breaking down & releasing energy − Anabolism: building up & requires energy ...
... and reproduce, maintain their structures, and respond to their environments. – Usually divided into two categories. Catabolism and Anabolism − Catabolism: breaking down & releasing energy − Anabolism: building up & requires energy ...
Cell Respiration DiagramSkit WS NEW
... Its role is to _____________________________ to form ______________ molecules, and has a net of ___ ATP molecules. ___________ molecules are also formed. 3. If oxygen is not present, the process goes into ________________ respiration, or _____________________. If oxygen is present, then the process ...
... Its role is to _____________________________ to form ______________ molecules, and has a net of ___ ATP molecules. ___________ molecules are also formed. 3. If oxygen is not present, the process goes into ________________ respiration, or _____________________. If oxygen is present, then the process ...
131110 COS ATP - Community of Reason
... transport chain (ETC) and ATP synthase. Oxidation-reduction reactions of the ETC sets up a proton gradient; energy “stored” in the proton gradient is converted to mechanical energy (rotation), which drives the synthesis of chemical energy (ATP). ATP is used to power cellular processes that require e ...
... transport chain (ETC) and ATP synthase. Oxidation-reduction reactions of the ETC sets up a proton gradient; energy “stored” in the proton gradient is converted to mechanical energy (rotation), which drives the synthesis of chemical energy (ATP). ATP is used to power cellular processes that require e ...
Metabolic Minimap article
... mitochondrial matrix with the exception of succinic dehydrogenase, which is part of Complex II of the inner membrane. It is important not to regard FADH2 as the product of this reaction, which is still often done. FAD is the first, but only a transient, carrier of electrons from succinate to ubiquin ...
... mitochondrial matrix with the exception of succinic dehydrogenase, which is part of Complex II of the inner membrane. It is important not to regard FADH2 as the product of this reaction, which is still often done. FAD is the first, but only a transient, carrier of electrons from succinate to ubiquin ...
How do cells regulate the speed of reactions?
... catabolism: breaking down organic molecules to extract energy Two Catabolic Pathways 1) Fermentation (anaerobic) 2) Cellular Respiration (aerobic) ...
... catabolism: breaking down organic molecules to extract energy Two Catabolic Pathways 1) Fermentation (anaerobic) 2) Cellular Respiration (aerobic) ...
Mattie Knebel Kyler Salazar Jared Hansen Biology 1610 Sperry
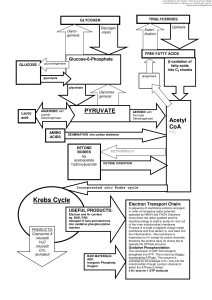
... 3 Carbon molecules called Pyruvate. This cycle takes place in the cytosol, located just outside of the mitochondria. This process has a net gain of 2 ATP which is then carried on to the next cycle. Diagram A, which can be found on our flow chart, shows this process. The 2nd cycle, the Citric Acid Cy ...
... 3 Carbon molecules called Pyruvate. This cycle takes place in the cytosol, located just outside of the mitochondria. This process has a net gain of 2 ATP which is then carried on to the next cycle. Diagram A, which can be found on our flow chart, shows this process. The 2nd cycle, the Citric Acid Cy ...
AP Biology
... 4. In cellular respiration, what is being oxidized and what is being reduced? oxidized – glucose ; reduced - oxygen 5. Label the diagram below of the electron movement with regard to the coenzyme NAD+. ...
... 4. In cellular respiration, what is being oxidized and what is being reduced? oxidized – glucose ; reduced - oxygen 5. Label the diagram below of the electron movement with regard to the coenzyme NAD+. ...
Cellular Respiration - Chapter 8 (new book).
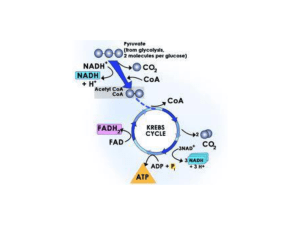
... 2. First intermediate formed is Citrate (Citric acid cycle) 3. Acetyl Co-A enters the cycle and bonds with oxaloacetate (C4) C6 4. 9 separate reactions 5. cycle occurs twice for each glucose metabolized 6. each cycle produces 2 CO2 molecules and 2 ATP (substrate level phosphorylation – enzyme cont ...
... 2. First intermediate formed is Citrate (Citric acid cycle) 3. Acetyl Co-A enters the cycle and bonds with oxaloacetate (C4) C6 4. 9 separate reactions 5. cycle occurs twice for each glucose metabolized 6. each cycle produces 2 CO2 molecules and 2 ATP (substrate level phosphorylation – enzyme cont ...
The ATP-PCr energy system can operate with or without oxygen but
... The aerobic system, which is dependent on oxygen, is the most complex of the three energy systems. The metabolic reactions that take place in the presence of oxygen are responsible for most of the cellular energy produced by the body. However, aerobic metabolism is the slowest way to resynthesize AT ...
... The aerobic system, which is dependent on oxygen, is the most complex of the three energy systems. The metabolic reactions that take place in the presence of oxygen are responsible for most of the cellular energy produced by the body. However, aerobic metabolism is the slowest way to resynthesize AT ...
p134
... (b) An electrochemical gradient is created during electron transport as the enzyme complexes move protons from NADH and FADH2 into the intermembrane space. The intermembrane space becomes an H+ reservoir because the membrane is almost impermeable to protons. There is, therefore, a much higher conce ...
... (b) An electrochemical gradient is created during electron transport as the enzyme complexes move protons from NADH and FADH2 into the intermembrane space. The intermembrane space becomes an H+ reservoir because the membrane is almost impermeable to protons. There is, therefore, a much higher conce ...
Electron Transport
... Electrons move along membrane from one protein to another. This release of electrons also causes NADH and FADH2 to lose a proton (H+) These protons are pumped into intermembrane space from the mitochondrial matrix ...
... Electrons move along membrane from one protein to another. This release of electrons also causes NADH and FADH2 to lose a proton (H+) These protons are pumped into intermembrane space from the mitochondrial matrix ...
Energy and Life - Lemon Bay High School
... • Energy is the ability to do work. • Cells are constantly using energy to perform LIFE FUNCTIONS. ...
... • Energy is the ability to do work. • Cells are constantly using energy to perform LIFE FUNCTIONS. ...
Energy and Life - Lemon Bay High School
... • Energy is the ability to do work. • Cells are constantly using energy to perform LIFE FUNCTIONS. ...
... • Energy is the ability to do work. • Cells are constantly using energy to perform LIFE FUNCTIONS. ...
Respiration - csfcA2Biology
... Energy can be released quickly, only one bond needs to be broken. Enzyme used: Bond broken: Energy per mole 30.6KJ ...
... Energy can be released quickly, only one bond needs to be broken. Enzyme used: Bond broken: Energy per mole 30.6KJ ...
Study Guide and Potential Essay Questions for Chapter 25
... hypothermia, Krebs’ cycle (TCA or citric acid cycle), lactic acid (lactate), metabolic rate, metabolic water, metabolism, minerals, mitochondrial matrix and inner membrane, NAD+/NADH + H+, nutrient, oxidation, oxidative phosphorylation, pyruvate-to-acetate step, reduction, substrate level phosphoryl ...
... hypothermia, Krebs’ cycle (TCA or citric acid cycle), lactic acid (lactate), metabolic rate, metabolic water, metabolism, minerals, mitochondrial matrix and inner membrane, NAD+/NADH + H+, nutrient, oxidation, oxidative phosphorylation, pyruvate-to-acetate step, reduction, substrate level phosphoryl ...
Review: Thermodynamics and Cell Respiration
... 18. What happens to the 6 carbon glucose molecule in aerobic respiration? Alcoholic fermentation? Lactic acid fermentation? ...
... 18. What happens to the 6 carbon glucose molecule in aerobic respiration? Alcoholic fermentation? Lactic acid fermentation? ...
METABOLISM OF CARBOHYDRATES
... V. ETP – electron transport pathway – oxidative phosphorylation (mitochondria) Released energy is stored in ATP molecules. These molecules store cellular energy needed to power cellular reactions. ATP ADP + P + 35 kJ/mole (8.4 kcal/mol) (4.184 J = 1 calorie) (turnover is very high estimates are ...
... V. ETP – electron transport pathway – oxidative phosphorylation (mitochondria) Released energy is stored in ATP molecules. These molecules store cellular energy needed to power cellular reactions. ATP ADP + P + 35 kJ/mole (8.4 kcal/mol) (4.184 J = 1 calorie) (turnover is very high estimates are ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.