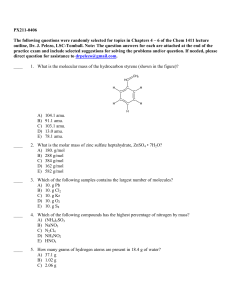
Unit 10 complete 2016-2017
... stain that can be used in paint. You want to do it as cheaply as possible. You now know that elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and he ...
... stain that can be used in paint. You want to do it as cheaply as possible. You now know that elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and he ...
The Oxidation States of Tin
... both compounds. The crystals for tin(IV) iodide weighed 0.4065 g and made up 73.8% of the total theoretical yield. The crystals from the tin(II) iodide experiment weighed 0.0293 g and made up 17.23% of the total theoretical yield. These crystals were meant to be differentiated from one another by th ...
... both compounds. The crystals for tin(IV) iodide weighed 0.4065 g and made up 73.8% of the total theoretical yield. The crystals from the tin(II) iodide experiment weighed 0.0293 g and made up 17.23% of the total theoretical yield. These crystals were meant to be differentiated from one another by th ...
AP Exam Review Questions
... • Ans: V2 = 250 ml then you must subtract 100 ml (the initial volume of KCl) because it takes up space in the flask, therefore the answer is volume of water = 150 ml ...
... • Ans: V2 = 250 ml then you must subtract 100 ml (the initial volume of KCl) because it takes up space in the flask, therefore the answer is volume of water = 150 ml ...
Problem 5. Inorganic chains and rings
... The list of topics of advanced difficulty The Safety rules and recommendations set by the IChO International Jury The hazard warning symbols, their designations and explanations, R-ratings and Sprovisions Worked solutions will be posted at the website by the end of May, 2013. We pay great atte ...
... The list of topics of advanced difficulty The Safety rules and recommendations set by the IChO International Jury The hazard warning symbols, their designations and explanations, R-ratings and Sprovisions Worked solutions will be posted at the website by the end of May, 2013. We pay great atte ...
Chemical Reactions - Effingham County Schools
... If 0.647 mol of oxygen is used in the burning of propane, how many moles each of CO2 and H2O are produced? How many moles of C3H8 are consumed? ...
... If 0.647 mol of oxygen is used in the burning of propane, how many moles each of CO2 and H2O are produced? How many moles of C3H8 are consumed? ...
picture_as_pdf Released Materials 2013 (16551
... Second World War came from galena, PbS(s). The following equations represent the reactions that are involved in refining galena to produce solid lead. Equation I Equation II Equation III ...
... Second World War came from galena, PbS(s). The following equations represent the reactions that are involved in refining galena to produce solid lead. Equation I Equation II Equation III ...
SQA Advanced Higher Chemistry Unit 2 Principles of Chemical
... Stoichiometry involves the understanding of the numerical relationships between reacting substances. It forms the basis for analytical chemistry. (How much iron is there in this sample of iron ore? How pure is this sample of anti-viral drug?) It is also critical for industrial chemistry. (How many t ...
... Stoichiometry involves the understanding of the numerical relationships between reacting substances. It forms the basis for analytical chemistry. (How much iron is there in this sample of iron ore? How pure is this sample of anti-viral drug?) It is also critical for industrial chemistry. (How many t ...
unit 7a homework packet - District 196 e
... 8. If excess ammonium sulfate reacts with 20.0 grams of calcium hydroxide, how many moles of ammonia (NH3) are produced? ___ (NH4)2SO4 + ___ Ca(OH)2 ...
... 8. If excess ammonium sulfate reacts with 20.0 grams of calcium hydroxide, how many moles of ammonia (NH3) are produced? ___ (NH4)2SO4 + ___ Ca(OH)2 ...
chem - CBSE Guess
... Rancidity: The oily and fatty food oxidizes and give bad smell and test is called rancidity.Preventatioin:By adding antioxidant which slow down the process of oxidation.2. Vaccum packing,3Flusing N2 gas in chips packets.3.Refrigeration. Q.Explain the various types of reactions with one example of ea ...
... Rancidity: The oily and fatty food oxidizes and give bad smell and test is called rancidity.Preventatioin:By adding antioxidant which slow down the process of oxidation.2. Vaccum packing,3Flusing N2 gas in chips packets.3.Refrigeration. Q.Explain the various types of reactions with one example of ea ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • In this type of reaction two or more substances react to form one product. • Examples: – 2 Mg (s) + O2 (g) 2 MgO (s) – N2 (g) + 3 H2 (g) 2 NH3 (g) – C3H6 (g) + Br2 (l) C3H6Br2 (l) ...
... • In this type of reaction two or more substances react to form one product. • Examples: – 2 Mg (s) + O2 (g) 2 MgO (s) – N2 (g) + 3 H2 (g) 2 NH3 (g) – C3H6 (g) + Br2 (l) C3H6Br2 (l) ...
Chemistry 3202 Grading Standards June 2006
... - treated the item as an indicator problem (i.e. used the indicator table to answer the question). - identified NO3G as a strong base; when base was added, the solution turned blue. - did not identify AgCl as the precipitate. (ii) When the equilibrium is placed in an ice bath it turns pale pink. Is ...
... - treated the item as an indicator problem (i.e. used the indicator table to answer the question). - identified NO3G as a strong base; when base was added, the solution turned blue. - did not identify AgCl as the precipitate. (ii) When the equilibrium is placed in an ice bath it turns pale pink. Is ...