Net ionic equation
... electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial posi ...
... electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial posi ...
CHE-310 Organic Chemistry I_
... CH4 + Br2 ------> HBr + CH3Br 2. Outline all steps in the mechanism for the above reaction, and calculate the delta H for each step in the mechanism. 3. Draw a graph of the potential energy changes in the second step of the above mechanism. (the graph can be free hand, but ...
... CH4 + Br2 ------> HBr + CH3Br 2. Outline all steps in the mechanism for the above reaction, and calculate the delta H for each step in the mechanism. 3. Draw a graph of the potential energy changes in the second step of the above mechanism. (the graph can be free hand, but ...
word - My eCoach
... calculations have correct units and correct number of significant digits. A student was tasked to determine the number of moles of water (n) in one mole of MgCl2·nH2O. She placed a small sample of MgCl2·nH2O in a dry crucible and heated it several times until all of the water has evaporated. From th ...
... calculations have correct units and correct number of significant digits. A student was tasked to determine the number of moles of water (n) in one mole of MgCl2·nH2O. She placed a small sample of MgCl2·nH2O in a dry crucible and heated it several times until all of the water has evaporated. From th ...
ExamView - 2002 AP Chemistry Exam.tst
... When the equation above is correctly balanced and all coefficients are reduced to lowest whole-number terms, the coefficient for H+(aq) is A) 2 B) 4 C) 6 D) 8 E) 14 50. Which of the following represents acceptable laboratory practice? A) Placing a hot object on a balance pan B) Using distilled water ...
... When the equation above is correctly balanced and all coefficients are reduced to lowest whole-number terms, the coefficient for H+(aq) is A) 2 B) 4 C) 6 D) 8 E) 14 50. Which of the following represents acceptable laboratory practice? A) Placing a hot object on a balance pan B) Using distilled water ...
Energy Matters - Perth Grammar
... An electron diagram to show the overlap of electron clouds in a water molecule is shown left. Draw a diagram to show electrons in the outer shells in the following molecules. ...
... An electron diagram to show the overlap of electron clouds in a water molecule is shown left. Draw a diagram to show electrons in the outer shells in the following molecules. ...
1.24 calculations and chemical reactions
... A solution of this acid was prepared by dissolving 2.02 g of H2A in water and making the volume up to 250 cm3 in a volumetric flask. A 25.0 cm3 sample of this solution required 22.80 cm3 of 0.150 mol dm–3 aqueous NaOH for complete reaction. Calculate the relative molecular mass, Mr, of H2A 4.2) Sodi ...
... A solution of this acid was prepared by dissolving 2.02 g of H2A in water and making the volume up to 250 cm3 in a volumetric flask. A 25.0 cm3 sample of this solution required 22.80 cm3 of 0.150 mol dm–3 aqueous NaOH for complete reaction. Calculate the relative molecular mass, Mr, of H2A 4.2) Sodi ...
Chemical reaction
... of the substances. These are called stoichiometric coefficients and represent the number ratio of element and/or compound across a balanced chemical equation. ...
... of the substances. These are called stoichiometric coefficients and represent the number ratio of element and/or compound across a balanced chemical equation. ...
Final Exam Review Packet
... - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quantity will be much smaller and a number that is easier to deal with than if you use grams or pounds. Also, you can compare two quantities of moles to each other, but you cannot compare grams ...
... - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quantity will be much smaller and a number that is easier to deal with than if you use grams or pounds. Also, you can compare two quantities of moles to each other, but you cannot compare grams ...
1 - A-Level Chemistry
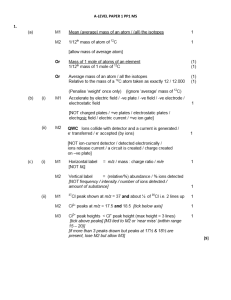
... Reagent and species can be extracted from the equation Ignore conditions such as dilute, concentrated, excess Reagent must be a compound NOT just an ion Equations must start from [Cu(H2O)6 ]2+ except in part (b) Mark reagent, species and equation independently ammonia (NH3) (solution) / NaOH ...
... Reagent and species can be extracted from the equation Ignore conditions such as dilute, concentrated, excess Reagent must be a compound NOT just an ion Equations must start from [Cu(H2O)6 ]2+ except in part (b) Mark reagent, species and equation independently ammonia (NH3) (solution) / NaOH ...
Chapter 4
... (10) Cancel species like H2O, OH-, or H+ that may appear on both sides. In this case, subtract 24 e-, 24 OH- and 12 H2O from each side. 3 N2H4 + 4 BrO3- → 4 Br- + 6 NO + 6 H2O (11) If necessary add spectator ions to get the balanced molecular equation. That is not possible in this case because we s ...
... (10) Cancel species like H2O, OH-, or H+ that may appear on both sides. In this case, subtract 24 e-, 24 OH- and 12 H2O from each side. 3 N2H4 + 4 BrO3- → 4 Br- + 6 NO + 6 H2O (11) If necessary add spectator ions to get the balanced molecular equation. That is not possible in this case because we s ...
4.6 M - Thierry Karsenti
... Charles’s law. The volume of a fixed amount of gas maintained at constant pressure is directly proportional to the absolute temperature. Closed system. A system with a boundary through which matter cannot be transferred. Dalton’s law. The total pressure of a mixture of gases is just a sum of the pre ...
... Charles’s law. The volume of a fixed amount of gas maintained at constant pressure is directly proportional to the absolute temperature. Closed system. A system with a boundary through which matter cannot be transferred. Dalton’s law. The total pressure of a mixture of gases is just a sum of the pre ...
Gas Laws
... unbalanced equation CH4 + O2 H2O + CO2 + CO. If 0.100 mol CH4 is allowed to react with 3.20 g O2, what is the limiting reagent, how many grams of carbon monoxide will be produced, and how many liters of the excess reactant will be left over when the reaction has gone to completion, assuming the re ...
... unbalanced equation CH4 + O2 H2O + CO2 + CO. If 0.100 mol CH4 is allowed to react with 3.20 g O2, what is the limiting reagent, how many grams of carbon monoxide will be produced, and how many liters of the excess reactant will be left over when the reaction has gone to completion, assuming the re ...
Gas Laws
... unbalanced equation CH4 + O2 H2O + CO2 + CO. If 0.100 mol CH4 is allowed to react with 3.20 g O2, what is the limiting reagent, how many grams of carbon monoxide will be produced, and how many liters of the excess reactant will be left over when the reaction has gone to completion, assuming the re ...
... unbalanced equation CH4 + O2 H2O + CO2 + CO. If 0.100 mol CH4 is allowed to react with 3.20 g O2, what is the limiting reagent, how many grams of carbon monoxide will be produced, and how many liters of the excess reactant will be left over when the reaction has gone to completion, assuming the re ...
Stoichiometry: Calculations with Chemical Formulas and
... – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined Stoichiometry ...
... – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined Stoichiometry ...
Exam 2
... Draw structural formulas for the following compounds. Give the molecular formula of each (CxHy.....). a) 2-methyl-3-hexene ...
... Draw structural formulas for the following compounds. Give the molecular formula of each (CxHy.....). a) 2-methyl-3-hexene ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e. react) during the course of the reaction. ...
... • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e. react) during the course of the reaction. ...