Thermodynamics and Equilibrium
... to conclude that processes should be spontaneous if they are exothermic. However, there are many examples of spontaneous endothermic processes, the most common being evaporation in which liquid molecules spontaneously break their intermolecular forces to pass into the gas phase. Thus, the enthalpy c ...
... to conclude that processes should be spontaneous if they are exothermic. However, there are many examples of spontaneous endothermic processes, the most common being evaporation in which liquid molecules spontaneously break their intermolecular forces to pass into the gas phase. Thus, the enthalpy c ...
Entropy (Part I)
... • In any spontaneous process, the path between reactants and products is irreversible. • Thermodynamics provides the direction of a process. It cannot predict the speed (rate) at which the process will occur. • Endothermic and exothermic reactions can both be spontaneous. ...
... • In any spontaneous process, the path between reactants and products is irreversible. • Thermodynamics provides the direction of a process. It cannot predict the speed (rate) at which the process will occur. • Endothermic and exothermic reactions can both be spontaneous. ...
Mole calculations File
... 3. Rinse a 250 ml beaker and a 250 cm3 volumetric flask with distilled water Why is this necessary? Why have I used ml & cm3? 4. Weigh a clean dry weighing boat (do not zero balance) 5. Add the mass Na2CO3 calculated in 2 (+/- 0.1 g ) 6. Tip into the beaker and reweigh the weighing boat ...
... 3. Rinse a 250 ml beaker and a 250 cm3 volumetric flask with distilled water Why is this necessary? Why have I used ml & cm3? 4. Weigh a clean dry weighing boat (do not zero balance) 5. Add the mass Na2CO3 calculated in 2 (+/- 0.1 g ) 6. Tip into the beaker and reweigh the weighing boat ...
1.24 calculations and chemical reactions
... 4.6) 1.00 cm3 of concentrated hydrochloric acid was transferred with a graduated pipette to a 100 cm3 volumetric flask. The volume was made up to 100 cm3 with distilled water. A 10.0 cm3 portion of the diluted solution from the volumetric flask was titrated by NaOH and was neutralised by 24.35 cm3 o ...
... 4.6) 1.00 cm3 of concentrated hydrochloric acid was transferred with a graduated pipette to a 100 cm3 volumetric flask. The volume was made up to 100 cm3 with distilled water. A 10.0 cm3 portion of the diluted solution from the volumetric flask was titrated by NaOH and was neutralised by 24.35 cm3 o ...
Chapter -
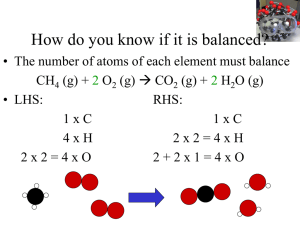
... 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 ...
... 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 ...
19_Worked_Examples
... (a) Cl2(g) is in its standard state, so ΔGf° is zero for this reactant. P4(g), however, is not in its standard state, so ΔGf° is not zero for this reactant. From the balanced equation and values from Appendix C, we have ΔG°rxn = 4 ΔGf°[PCl3(g)] − ΔGf°[P4(g)] − 6 ΔGf°[Cl2(g)] = (4 mol)(−269.6 kJ ⁄ mo ...
... (a) Cl2(g) is in its standard state, so ΔGf° is zero for this reactant. P4(g), however, is not in its standard state, so ΔGf° is not zero for this reactant. From the balanced equation and values from Appendix C, we have ΔG°rxn = 4 ΔGf°[PCl3(g)] − ΔGf°[P4(g)] − 6 ΔGf°[Cl2(g)] = (4 mol)(−269.6 kJ ⁄ mo ...
LN_ch06
... factors used in problem solving. These conversion factors are called “mole ratios.” 4Fe (s) + 3O2 (g) 2Fe2O3 (s) ...
... factors used in problem solving. These conversion factors are called “mole ratios.” 4Fe (s) + 3O2 (g) 2Fe2O3 (s) ...
Topic 1: Quantitative chemistry
... oxygen. The compound forms a white precipitate when it reacts with barium nitrate solution. Topic1notes 12.5 hours Page 7 of 31 ...
... oxygen. The compound forms a white precipitate when it reacts with barium nitrate solution. Topic1notes 12.5 hours Page 7 of 31 ...
EQUILIBRIUM
... [H2] = [I2] = 0.10 M 1 point for stoichiometric relationship between HI and H2 and I2 1 point for equilibrium concentrations of H2 and I2 d. From the graph, [H2] eq is 0.10 M. The curve should have the following characteristics: start at 0 M, increase to 0.10 M, reach equilibrium at the same time [H ...
... [H2] = [I2] = 0.10 M 1 point for stoichiometric relationship between HI and H2 and I2 1 point for equilibrium concentrations of H2 and I2 d. From the graph, [H2] eq is 0.10 M. The curve should have the following characteristics: start at 0 M, increase to 0.10 M, reach equilibrium at the same time [H ...
EQUILIBRIUM
... [H2] = [I2] = 0.10 M 1 point for stoichiometric relationship between HI and H2 and I2 1 point for equilibrium concentrations of H2 and I2 d. From the graph, [H2] eq is 0.10 M. The curve should have the following characteristics: start at 0 M, increase to 0.10 M, reach equilibrium at the same time [H ...
... [H2] = [I2] = 0.10 M 1 point for stoichiometric relationship between HI and H2 and I2 1 point for equilibrium concentrations of H2 and I2 d. From the graph, [H2] eq is 0.10 M. The curve should have the following characteristics: start at 0 M, increase to 0.10 M, reach equilibrium at the same time [H ...
Energy is the essence of chemistry It determines which reaction can
... Calculation when reactant is not 1.0 mol. Example Calculate the heat for combustion of 10.0g of CH3OH(l) o at constant pressure if ∆H r = -363 kJ/mol Solution: The combustion reaction is: CH3OH(l) + 3/2O2(g) → CO2(g) + 1/2H2O(l) q = nx ∆H r Must convert 10.0g to number of moles n. nCH3OH = 10.0g⋅(1m ...
... Calculation when reactant is not 1.0 mol. Example Calculate the heat for combustion of 10.0g of CH3OH(l) o at constant pressure if ∆H r = -363 kJ/mol Solution: The combustion reaction is: CH3OH(l) + 3/2O2(g) → CO2(g) + 1/2H2O(l) q = nx ∆H r Must convert 10.0g to number of moles n. nCH3OH = 10.0g⋅(1m ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... An active science program presents some hazards to both staff and students. All attempts will be made however, to identify hazards and manage risks so that they become minimal. Before each activity, instructions will be given to reduce any risks. Teachers will assess the readiness level of students ...
... An active science program presents some hazards to both staff and students. All attempts will be made however, to identify hazards and manage risks so that they become minimal. Before each activity, instructions will be given to reduce any risks. Teachers will assess the readiness level of students ...
Enthalpy Moles Notes - Chemistry Teaching Resources
... Notice, again, that there is often a need to use ‘fractions’ of moles in order to balance these equations in terms of one mole of water ,as defined. Notice that enthalpies of neutralisation are always exothermic, ∆H negative, and that the value is the same for many combinations of acids / alkalis. Th ...
... Notice, again, that there is often a need to use ‘fractions’ of moles in order to balance these equations in terms of one mole of water ,as defined. Notice that enthalpies of neutralisation are always exothermic, ∆H negative, and that the value is the same for many combinations of acids / alkalis. Th ...
CHAPTER I
... Copper, in Group IB, will also have one electron assigned to the 4s orbital, plus 28 other electrons assigned to other orbitals. The configuration of Be 1s2 2s2.All elements of Group 2A have electron configurations [electrons of preceding rare gas + ns2], where n is the period in which the element ...
... Copper, in Group IB, will also have one electron assigned to the 4s orbital, plus 28 other electrons assigned to other orbitals. The configuration of Be 1s2 2s2.All elements of Group 2A have electron configurations [electrons of preceding rare gas + ns2], where n is the period in which the element ...