Line 4: Equation
... 7. Calculate the mole ratio. In order to get a ratio, you have to divide two numbers that have the same unit. We were given moles, so we can divide line 2 by line 6. If we were given grams, we would divide the given number on line 3 by line 7. Write this number next to mole ratio. 2.5 mol/ 1 mol = 2 ...
... 7. Calculate the mole ratio. In order to get a ratio, you have to divide two numbers that have the same unit. We were given moles, so we can divide line 2 by line 6. If we were given grams, we would divide the given number on line 3 by line 7. Write this number next to mole ratio. 2.5 mol/ 1 mol = 2 ...
Chemistry 11 Lab booklet # ___
... change along with proof of this evidence from the lab. Use an example or two from the lab that shows the sign of chemical change in action. ConclusionList all the different signs of chemical change but there is no need to state evidence like in the analysis. Evaluation: a) Sources of possible errors ...
... change along with proof of this evidence from the lab. Use an example or two from the lab that shows the sign of chemical change in action. ConclusionList all the different signs of chemical change but there is no need to state evidence like in the analysis. Evaluation: a) Sources of possible errors ...
____ 1. The energy required to convert a ground
... 45. The graph above shows the results of a study of the reaction of X with a large excess of Y to yield Z. The concentrations of X and Y were measured over a period of time. According to the results, which of the following can be concluded about the rate law for the reaction under the conditions stu ...
... 45. The graph above shows the results of a study of the reaction of X with a large excess of Y to yield Z. The concentrations of X and Y were measured over a period of time. According to the results, which of the following can be concluded about the rate law for the reaction under the conditions stu ...
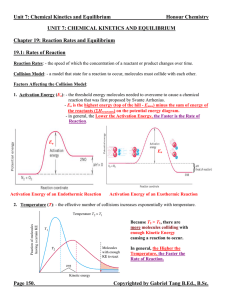
Unit 7 Reaction Rates and Equilibrium Notes
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
Chemical Reaction Stoichiometry (CRS): A Tutorial
... Chemical reaction stoichiometry (CRS) is a branch of chemical stoichiometry dealing with the constraints, in the form of chemical equations, placed on changes in the composition of a closed reacting system by the requirement for conservation of the amount of each atomic species and of the total char ...
... Chemical reaction stoichiometry (CRS) is a branch of chemical stoichiometry dealing with the constraints, in the form of chemical equations, placed on changes in the composition of a closed reacting system by the requirement for conservation of the amount of each atomic species and of the total char ...
Test
... They are much smaller. b) They are much bigger. c) They are about the same. d) They have to be exactly equal. e) You can't tell from the information given. ...
... They are much smaller. b) They are much bigger. c) They are about the same. d) They have to be exactly equal. e) You can't tell from the information given. ...
Basic Concepts
... pressures are substituted into the equilibrium constant expression for the reaction. • When equilibrium concentrations are not given the equilibrium concentrations can be obtained from the initial concentrations of the reactants and the balanced equation for the reaction, as long as the equilibrium ...
... pressures are substituted into the equilibrium constant expression for the reaction. • When equilibrium concentrations are not given the equilibrium concentrations can be obtained from the initial concentrations of the reactants and the balanced equation for the reaction, as long as the equilibrium ...
Name_________________________________________
... 1. How many grams of PbO are consumed in the reaction of 8.16 g PbO? (MM of PbO is 223.2 g/mol) 2. If 0.312 g of NH3 is available to react with 8.16 g PbO, how many grams of Pb are produced? 3. If the actual yield is 4.95 g Pb, what is the percent yield? [ANS = 0.415 g, 5.69 g, 87%] Benzocaine is a ...
... 1. How many grams of PbO are consumed in the reaction of 8.16 g PbO? (MM of PbO is 223.2 g/mol) 2. If 0.312 g of NH3 is available to react with 8.16 g PbO, how many grams of Pb are produced? 3. If the actual yield is 4.95 g Pb, what is the percent yield? [ANS = 0.415 g, 5.69 g, 87%] Benzocaine is a ...
honors chemistry harvard-westlake second semester final exam
... How are you going to prepare for the Final Exam? The best preparation for the final exam is consistent study habits and effort throughout the year. We know that a lot of material has gone down the lab sink, so to speak, and so we also have some suggestions for areas you should concentrate on as you ...
... How are you going to prepare for the Final Exam? The best preparation for the final exam is consistent study habits and effort throughout the year. We know that a lot of material has gone down the lab sink, so to speak, and so we also have some suggestions for areas you should concentrate on as you ...
Chem. 1310 Fall 2005 Final Exam-white ... Name _________________________________ Section Number ___________________
... a. increase the entropy of the universe. b. decrease the energy of the universe. Answer: a 22. Which of the following are generally true? a. Intermolecular forces are weaker than covalent bonds. b. Intermolecular forces are more directional than covalent bonds. c. Any molecule in a gas experiences i ...
... a. increase the entropy of the universe. b. decrease the energy of the universe. Answer: a 22. Which of the following are generally true? a. Intermolecular forces are weaker than covalent bonds. b. Intermolecular forces are more directional than covalent bonds. c. Any molecule in a gas experiences i ...
In Situ Soft X‑ray Absorption Spectroscopy Applied to Solid
... to π*CO orbital in the amide group has been reported in various amide compounds, e.g., formamide45 and malonamide.46 In Figure 2, a very small absorption peak corresponding to π*CO is also observed around 400 eV.15 All the absorption peaks observed in the C K-edge XAS (Figure 1) and N K-edge XAS ( ...
... to π*CO orbital in the amide group has been reported in various amide compounds, e.g., formamide45 and malonamide.46 In Figure 2, a very small absorption peak corresponding to π*CO is also observed around 400 eV.15 All the absorption peaks observed in the C K-edge XAS (Figure 1) and N K-edge XAS ( ...
Mathematical Modeling of the Formation of Calcareous
... Cathodic protection (CP) has been recognized as an effective method for preventing immersed offshore structures from corroding. Under cathodic protection, the oxidation of iron is prohibited by supplying electrons to the metal structure to be protected by means of sacrificial anodes or impressed cur ...
... Cathodic protection (CP) has been recognized as an effective method for preventing immersed offshore structures from corroding. Under cathodic protection, the oxidation of iron is prohibited by supplying electrons to the metal structure to be protected by means of sacrificial anodes or impressed cur ...
Chapter 3
... substance before the → are reactants (starting materials) and the substances after the → are the products. In all chemical reactions some number may occurs in front of the reactant and the products (if the number is 1 we ignore it as it its understandable without being written), these numbers indica ...
... substance before the → are reactants (starting materials) and the substances after the → are the products. In all chemical reactions some number may occurs in front of the reactant and the products (if the number is 1 we ignore it as it its understandable without being written), these numbers indica ...
Empirical Formula, Molecular Formula, Percent Composition
... Compare both of your reactant amounts to the same product in this case Al2(SO4)3. Then find out how much products will be produced from each individual reactant. Whichever reactant yields the least amount of product that is your limiting reactant. 4 moles Al x 1 mole Al2(SO4)3 / 2 moles Al= 2 moles ...
... Compare both of your reactant amounts to the same product in this case Al2(SO4)3. Then find out how much products will be produced from each individual reactant. Whichever reactant yields the least amount of product that is your limiting reactant. 4 moles Al x 1 mole Al2(SO4)3 / 2 moles Al= 2 moles ...
The Mole - C405 Chemistry
... C.8.E Perform stoichiometric calculations, including determination of mass relationships between reactants and products, calculation of limiting reagents, and percent yield ...
... C.8.E Perform stoichiometric calculations, including determination of mass relationships between reactants and products, calculation of limiting reagents, and percent yield ...