Chemistry 3202 Grading Standards June 2006
... - treated the item as an indicator problem (i.e. used the indicator table to answer the question). - identified NO3G as a strong base; when base was added, the solution turned blue. - did not identify AgCl as the precipitate. (ii) When the equilibrium is placed in an ice bath it turns pale pink. Is ...
... - treated the item as an indicator problem (i.e. used the indicator table to answer the question). - identified NO3G as a strong base; when base was added, the solution turned blue. - did not identify AgCl as the precipitate. (ii) When the equilibrium is placed in an ice bath it turns pale pink. Is ...
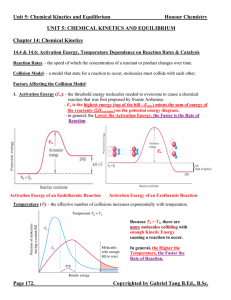
5.2 Calculations of Enthalpy Changes (SL/HL)
... Energy is required to break bonds. Energy is released when bonds form. In an exothermic reaction, the amount of energy required to break the bonds of the reactants is less then the amount of energy released when the bonds form in the products. Enthalpy ...
... Energy is required to break bonds. Energy is released when bonds form. In an exothermic reaction, the amount of energy required to break the bonds of the reactants is less then the amount of energy released when the bonds form in the products. Enthalpy ...
Formation Mechanisms of Naphthalene and
... reactions arising from the great variety of molecules and radicals that are present in different isomeric forms.12−15 The reactions involved occur over intricate potential energy surfaces with multiple local minima and products and hence their mechanisms, rates, and product yields may strongly depend ...
... reactions arising from the great variety of molecules and radicals that are present in different isomeric forms.12−15 The reactions involved occur over intricate potential energy surfaces with multiple local minima and products and hence their mechanisms, rates, and product yields may strongly depend ...
CHEMISTRY - careerpoint.ac.in
... never exist. Therefore, 1 mole of those metals represented by the weight of NA number of such metal atoms and thus, 1 mole of the given substances is equal to gram atomic weight of those metals. (f) Mole concept is based on the application of the law of conservation of atoms, first proposed by Dalto ...
... never exist. Therefore, 1 mole of those metals represented by the weight of NA number of such metal atoms and thus, 1 mole of the given substances is equal to gram atomic weight of those metals. (f) Mole concept is based on the application of the law of conservation of atoms, first proposed by Dalto ...
Chemical Equilibrium
... Consider the following reaction occurring in a closed container (so that no material can go in or out): H2 + I2 → 2HI This is simply the reaction between elemental hydrogen and elemental iodine to make hydrogen iodide. The way the equation is written, we are led to believe that the reaction goes to ...
... Consider the following reaction occurring in a closed container (so that no material can go in or out): H2 + I2 → 2HI This is simply the reaction between elemental hydrogen and elemental iodine to make hydrogen iodide. The way the equation is written, we are led to believe that the reaction goes to ...
Physical Chemistry 1.pdf
... calorimeter to the surroundings. It is used to measure the heat of a combustion reaction. The measured heat of reaction at constant volume, qv = ΔE. Boyle’s law. The volume of a fixed mass of a gas maintained at constant temperature is inversely proportional to the gas pressure. Calorimeter. A devic ...
... calorimeter to the surroundings. It is used to measure the heat of a combustion reaction. The measured heat of reaction at constant volume, qv = ΔE. Boyle’s law. The volume of a fixed mass of a gas maintained at constant temperature is inversely proportional to the gas pressure. Calorimeter. A devic ...
UNIVERSITY OF TARTU THE GIFTED AND
... water. In one beaker, the amount of water displaced by the gas was two times the amount displaced in the other beaker. a) Write the equations for the half-reactions for both the anode and cathode. Write the equation for the overall reaction. b) Calculate: i) the masses of the substances formed durin ...
... water. In one beaker, the amount of water displaced by the gas was two times the amount displaced in the other beaker. a) Write the equations for the half-reactions for both the anode and cathode. Write the equation for the overall reaction. b) Calculate: i) the masses of the substances formed durin ...
Word - icho39.chem.msu.ru
... 1. In 1875 the French chemist Paul-Emile Lecoq de Boisbaudran studied the spectra of zinc ore and discovered the traces of a new element, which he called “gallium” from the Latin word "Gallia" meaning "France" and perhaps also from the Latin word "gallus" (the cock, a translation of Lecoq). In the s ...
... 1. In 1875 the French chemist Paul-Emile Lecoq de Boisbaudran studied the spectra of zinc ore and discovered the traces of a new element, which he called “gallium” from the Latin word "Gallia" meaning "France" and perhaps also from the Latin word "gallus" (the cock, a translation of Lecoq). In the s ...
Personal Tutoring Help on Questions and Problems
... 3.44 Peroxyacylnitrate (PAN) is one of the components of smog. It is a compound of C, H, N, and O. Determine * the percent composition of oxygen and the empirical formula from the following percent composition by mass: 19.8 percent C, 2.50 percent H, 11.6 percent N. What is its molecular formula giv ...
... 3.44 Peroxyacylnitrate (PAN) is one of the components of smog. It is a compound of C, H, N, and O. Determine * the percent composition of oxygen and the empirical formula from the following percent composition by mass: 19.8 percent C, 2.50 percent H, 11.6 percent N. What is its molecular formula giv ...
Topic 5 Energetics File
... Enthalpy: The internal energy stored in the reactants. Only changes in enthalpy can be measured. Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) incre ...
... Enthalpy: The internal energy stored in the reactants. Only changes in enthalpy can be measured. Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) incre ...
AP Ch 3 Stoichiometry
... n x molar mass of element x 100% molar mass of compound n is the number of moles of the element in 1 mole of the compound 2 x (12.01 g) x 100% = 52.14% 46.07 g 6 x (1.008 g) %H = x 100% = 13.13% 46.07 g 1 x (16.00 g) %O = x 100% = 34.73% 46.07 g ...
... n x molar mass of element x 100% molar mass of compound n is the number of moles of the element in 1 mole of the compound 2 x (12.01 g) x 100% = 52.14% 46.07 g 6 x (1.008 g) %H = x 100% = 13.13% 46.07 g 1 x (16.00 g) %O = x 100% = 34.73% 46.07 g ...
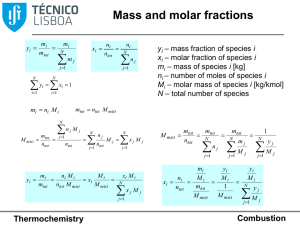
Mass Relationships in Chemical Reactions
... Many tons of urea (CO(NH2)2) are produced every year in fertilizer industries. When 119 g ammonia react with 80 g CO2 as the equation: 2 NH 3 (g ) + CO 2 (g ) → CO( NH 2 ) 2 (s ) + H 2O and produce 100 g urea, calculate % yield? - Step 1: Determine the limiting reagent - Change the mass in grams int ...
... Many tons of urea (CO(NH2)2) are produced every year in fertilizer industries. When 119 g ammonia react with 80 g CO2 as the equation: 2 NH 3 (g ) + CO 2 (g ) → CO( NH 2 ) 2 (s ) + H 2O and produce 100 g urea, calculate % yield? - Step 1: Determine the limiting reagent - Change the mass in grams int ...
Chemistry Standard Level Chapter 1
... Equal quantities of apples and oranges do not have equal masses or equal volumes but equal numbers. The chemist adopts the same approach. As all matter is made up from small particles (see Chapter 2), we measure amount by counting particles. If the substance is an element we usually count atoms, if ...
... Equal quantities of apples and oranges do not have equal masses or equal volumes but equal numbers. The chemist adopts the same approach. As all matter is made up from small particles (see Chapter 2), we measure amount by counting particles. If the substance is an element we usually count atoms, if ...
1 Quantitative chemistry - Pearson Schools and FE Colleges
... Equal quantities of apples and oranges do not have equal masses or equal volumes but equal numbers. The chemist adopts the same approach. As all matter is made up from small particles (see Chapter 2), we measure amount by counting particles. If the substance is an element we usually count atoms, if ...
... Equal quantities of apples and oranges do not have equal masses or equal volumes but equal numbers. The chemist adopts the same approach. As all matter is made up from small particles (see Chapter 2), we measure amount by counting particles. If the substance is an element we usually count atoms, if ...